Analytical Chemistry Exam Questions and Answers
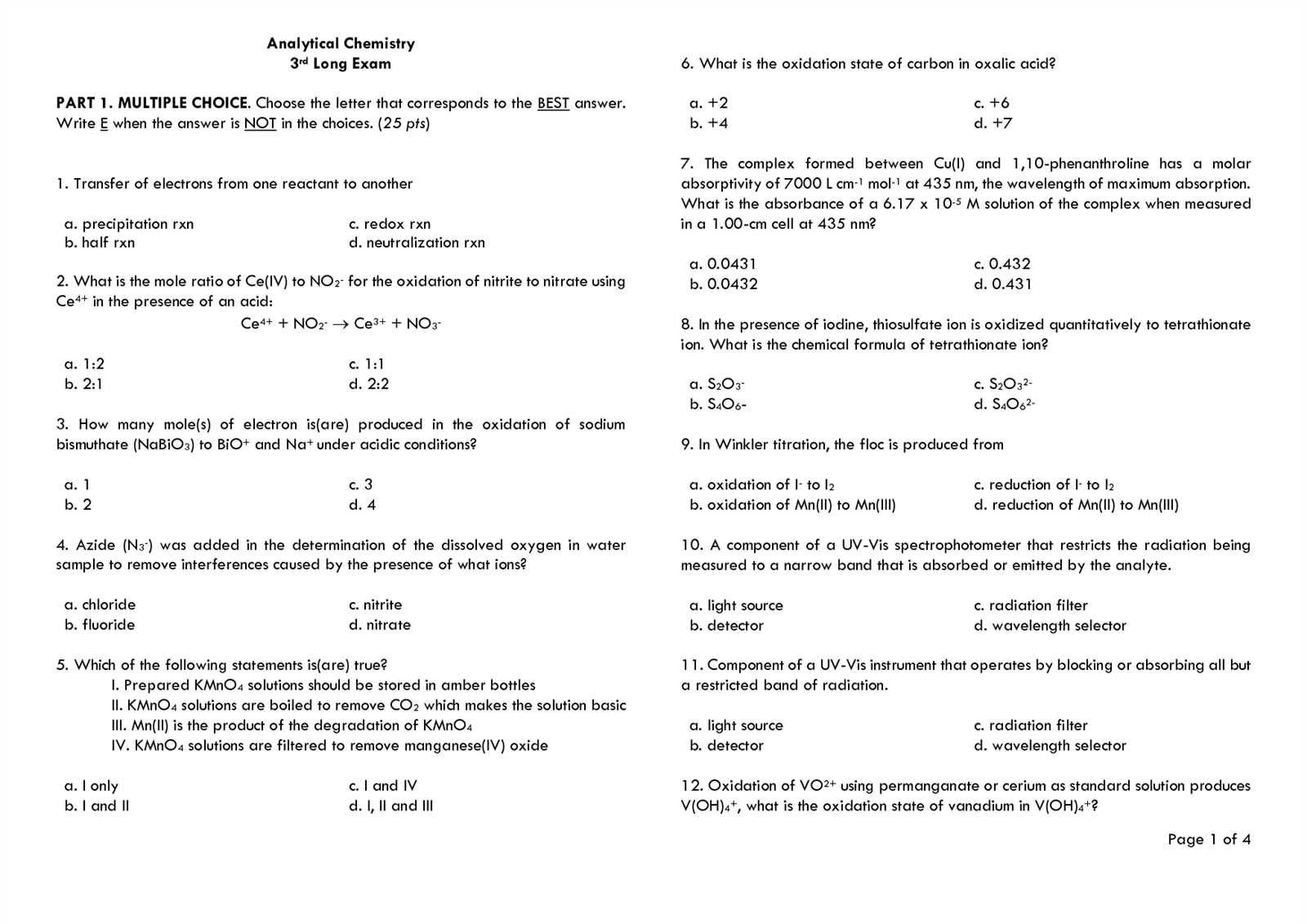
Success in scientific assessments relies heavily on your ability to understand fundamental concepts, apply techniques, and solve problems efficiently. Whether you’re dealing with chemical processes, lab apparatus, or data analysis, thorough preparation is key to mastering the material.
To excel, it’s essential to focus on key principles, develop strong problem-solving skills, and practice interpreting results. Practicing with real-world scenarios will give you an edge, helping you approach any challenge with confidence and clarity.
Strategic review plays a significant role in reinforcing knowledge. By analyzing past evaluations and addressing common areas of difficulty, you can ensure that you’re well-prepared for any test. Developing a methodical study routine will allow you to master all essential topics effectively.
Remember, consistent practice, along with a clear understanding of various techniques and approaches, will make a significant difference in your performance. Stay focused, and use the resources available to you to achieve the best results.
Analytical Chemistry Exam Questions and Answers
When preparing for a scientific assessment, it’s crucial to understand the fundamental concepts, techniques, and methods that will likely be tested. Mastering these principles will help you navigate various topics with confidence, from identifying compounds to interpreting experimental data.
By reviewing common problems from previous assessments, you can familiarize yourself with the types of tasks you may encounter. This approach not only improves your problem-solving skills but also boosts your ability to apply theory to real-world situations.
Effective preparation involves both theoretical understanding and practical application. Practicing with example scenarios allows you to approach complex challenges systematically and develop the ability to analyze results accurately.
Focus on key areas such as measurements, data analysis, and laboratory techniques. Understanding these concepts deeply will enhance your ability to answer questions efficiently and correctly during your test.
Understanding the Basics of Analytical Chemistry
A solid foundation in scientific techniques is essential for anyone looking to succeed in a laboratory-based environment. This involves understanding core principles such as measurements, the use of laboratory instruments, and the interpretation of experimental data. Mastery of these topics forms the backbone of successful problem-solving and accurate results.
At the core of these practices is the ability to distinguish between different substances, identify their properties, and analyze their behavior in various conditions. A strong grasp of these elements allows you to handle complex processes and provides the groundwork for more advanced studies.
Here’s a summary of key concepts that every student should be familiar with:
| Concept | Description |
|---|---|
| Measurement Techniques | Methods used to determine the physical properties of substances, such as mass, volume, or concentration. |
| Instrumental Methods | Utilizing tools like spectrometers or chromatographs to analyze samples. |
| Data Interpretation | Evaluating experimental results to draw meaningful conclusions and ensure accuracy. |
| Experimental Design | Planning experiments in a way that minimizes errors and ensures reproducibility. |
By mastering these fundamental areas, you’ll be well-prepared to tackle more complex challenges and perform rigorous analysis in the laboratory. With practice, these skills become second nature and provide a strong foundation for any scientific study.
Key Topics to Study for Exams
Effective preparation for any scientific assessment requires a clear focus on the core topics that are likely to appear. To perform well, it is essential to understand the main concepts, tools, and methods used in the field. By targeting these key areas, you can build a strong foundation and tackle the most common challenges efficiently.
Fundamental Concepts
Familiarizing yourself with the basic principles is the first step toward mastering the subject. Focus on topics that form the foundation of most tests:
- Measurement techniques and units
- Sample preparation and handling
- Understanding various substances and their properties
- Analyzing data from experiments
Practical Techniques
In addition to theory, it’s crucial to understand the hands-on methods used in the laboratory. Some of the key techniques to focus on include:
- Using laboratory instruments effectively
- Performing titrations and calibrations
- Chromatography and spectroscopy methods
- Data interpretation and error analysis
By thoroughly studying these areas, you’ll be better equipped to address both theoretical and practical challenges. Review your notes regularly, work through practice problems, and familiarize yourself with real-world applications of these concepts. With dedication, you will improve your performance and feel confident during the assessment.
Common Analytical Techniques in Chemistry

In scientific studies, several methods are commonly employed to identify, measure, and analyze substances. These techniques are fundamental for obtaining accurate results, and they play a critical role in laboratory work, research, and industrial applications. Mastering these methods ensures the ability to conduct precise investigations and draw meaningful conclusions from experimental data.
Among the most frequently used techniques are:
- Spectroscopy – This method involves the interaction of light with matter to determine the composition of substances. Various types of spectroscopy, such as UV-Vis, IR, and NMR, are used depending on the sample and the type of analysis required.
- Chromatography – A separation technique that allows for the analysis of complex mixtures by separating their components. Common forms include gas and liquid chromatography, which are essential for purity testing and compound identification.
- Titration – This technique is used to determine the concentration of a substance in a solution by reacting it with a known volume of another solution. It is commonly applied in acid-base and redox reactions.
- Electrochemical Methods – Techniques like potentiometry and voltammetry are used to measure the potential or current in a solution, helping to identify the concentration of ions or detect specific chemical reactions.
Each technique serves a unique purpose, providing different insights into the properties and behavior of substances. Understanding how and when to apply these methods is essential for any scientific study or laboratory investigation.
Preparing for Instrumentation Questions
In many assessments, questions related to laboratory tools and techniques play a significant role. These questions typically focus on understanding how different instruments work, their applications, and the data they produce. A solid grasp of how to operate and interpret results from these tools is essential for success.
To effectively prepare for this section, it’s important to focus on both the theoretical principles behind the instruments and their practical use in experiments. Key areas to review include:
- Types of Instruments – Learn about the various tools used in the lab, such as spectrometers, chromatographs, and titration setups. Understand their functions and when each is most appropriate.
- Operating Principles – Familiarize yourself with how these instruments work, including the technical principles that govern their operation. Knowing how to set them up and calibrate them is crucial.
- Data Interpretation – Be prepared to analyze the results produced by different devices. This includes understanding how to interpret graphs, spectra, and other forms of data that instruments provide.
- Common Troubleshooting – Understand typical errors that might occur with instruments and how to address them, including calibration issues or data inconsistencies.
By thoroughly reviewing these topics, you can increase your ability to handle instrumentation-related tasks in assessments. Practice with real-world scenarios to enhance your understanding of both theory and practice, ensuring a well-rounded preparation.
Organic Chemistry Questions in Exams
In many assessments, a significant portion of the content revolves around the study of carbon-based compounds, their structures, reactions, and synthesis methods. Understanding the behavior of these compounds is essential, as it forms the foundation for numerous real-world applications, including pharmaceuticals, agriculture, and environmental science. Mastering these topics is crucial for success in any scientific evaluation.
When preparing for these sections, focus on the following key areas:
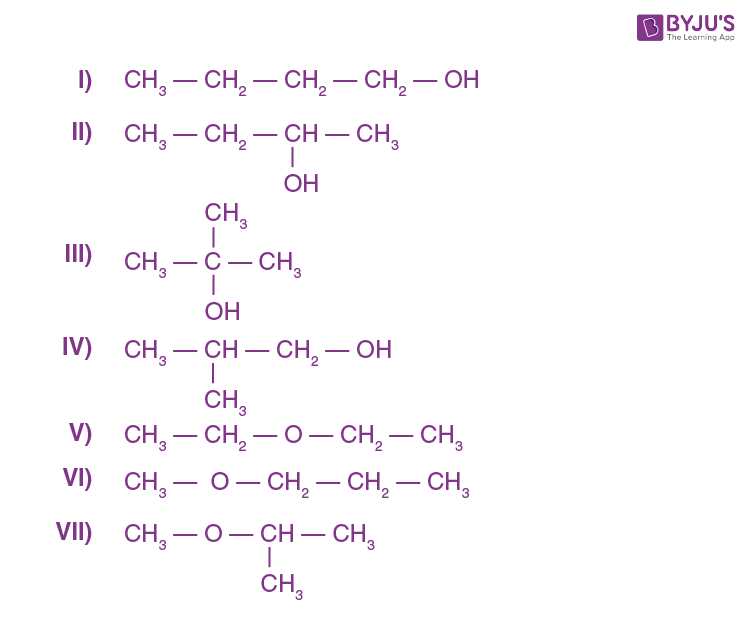
- Functional Groups – Recognize and understand the reactivity of various functional groups, such as alcohols, aldehydes, ketones, and carboxylic acids. Knowing how these groups interact is vital for answering related problems.
- Reaction Mechanisms – Study the pathways through which chemical reactions occur, including nucleophilic substitutions, electrophilic additions, and elimination reactions. Be prepared to explain each step of the mechanism and predict products.
- Synthesis Techniques – Understand how to design synthetic routes for creating complex molecules from simpler ones. This includes understanding reagents, catalysts, and reaction conditions.
- Spectroscopic Identification – Be familiar with methods like IR, NMR, and mass spectrometry used to identify and confirm the structures of organic compounds.
To excel in these topics, practice applying the principles to a variety of scenarios. Work through example problems, study reaction pathways, and familiarize yourself with the types of compounds commonly tested. By reinforcing your knowledge, you can approach any question with confidence.
Inorganic Chemistry Concepts You Should Know
In many scientific assessments, a deep understanding of the behavior of non-organic compounds is crucial. This area of study includes the properties, reactions, and classification of elements and their compounds. Mastering these concepts is essential for addressing complex problems related to metal ions, salts, and the periodic trends of various elements.
Key Topics to Focus On
To effectively prepare, focus on the fundamental principles that underpin this branch of science. Some of the most important topics include:
- Periodic Table Trends – Understand how the properties of elements change across periods and groups, including trends in electronegativity, ionization energy, and atomic radius.
- Oxidation-Reduction Reactions – Be prepared to identify oxidation states and balance redox reactions. This is crucial for understanding many reactions involving metals and non-metals.
- Coordination Compounds – Study the structure, bonding, and reactions of coordination complexes, including ligands, central atoms, and coordination numbers.
- Acid-Base Theories – Know the various theories that describe acid-base reactions, such as the Arrhenius, Bronsted-Lowry, and Lewis models.
Practical Applications
These concepts are not only academic but also have practical applications in various fields, including material science, environmental science, and industrial processes. Understanding how different compounds interact in real-world settings will enhance your ability to solve complex problems and interpret experimental data.
Common Mistakes in Analytical Chemistry Exams
During assessments in scientific subjects, students often encounter specific challenges that lead to mistakes, which can significantly impact their performance. These errors usually stem from misunderstanding core concepts, misapplying techniques, or overlooking crucial details in problem-solving. Recognizing and avoiding these common pitfalls can improve accuracy and boost scores.
Some of the most frequent mistakes include:
- Misinterpreting Data – One of the most common issues is incorrectly analyzing or interpreting experimental results. Always ensure that units, measurements, and calculations are double-checked before drawing conclusions.
- Ignoring Significant Figures – Failing to follow proper rules for significant figures when performing calculations can lead to imprecise results, affecting the overall quality of answers.
- Overlooking Assumptions – Many problems involve assumptions or ideal conditions, such as constant temperature or pressure. Forgetting to account for these factors can lead to incorrect answers.
- Skipping Units – Not including or converting units correctly is another common mistake. Ensure that all units are consistently used throughout calculations and that conversions are properly made where necessary.
- Rushing Through Reactions – Incomplete or incorrect reaction mechanisms are common when students rush through steps. Take time to carefully explain each stage of a reaction to avoid missing important details.
By being aware of these frequent errors and practicing careful, methodical problem-solving techniques, students can enhance their performance and improve their understanding of the subject matter.
How to Tackle Data Interpretation Questions

When confronted with questions involving data analysis, the key is to approach them systematically. These types of problems often require you to extract meaningful information from tables, graphs, or experimental results. Understanding the context of the data and identifying the most relevant details is essential for providing accurate responses.
Here’s a step-by-step guide on how to approach these types of questions:
- Understand the Data – Before jumping into calculations or interpretations, carefully examine the data presented. Identify the variables, units, and any trends that are immediately apparent.
- Identify Key Information – Focus on the most important points that are necessary to answer the question. For example, if asked about the relationship between two variables, look for patterns or correlations in the data.
- Look for Trends or Outliers – Pay attention to trends such as increases, decreases, or constancy over time. Outliers or unexpected data points can often provide important clues for analysis.
- Use Appropriate Calculations – If the question requires calculations, make sure to follow the correct formulas and include the necessary units. Double-check your calculations for accuracy.
- Relate the Data to Theory – Connect your observations and results to relevant scientific concepts. This helps ensure that your interpretations are grounded in established principles.
For example, when interpreting experimental results, you might be given a table like the following:
| Concentration (mol/L) | Absorbance |
|---|---|
| 0.1 | 0.15 |
| 0.2 | 0.30 |
| 0.3 | 0.45 |
From this table, you can identify a direct relationship between concentration and absorbance. By applying the relevant formula, you could calculate the concentration based on absorbance readings or determine the slope of the line for further analysis.
In summary, effective data interpretation requires careful observation, logical reasoning, and an understanding of the principles that govern the results. Practice with various data sets will help sharpen your skills and improve your confidence in tackling such questions.
Strategies for Solving Chemical Equations

Solving chemical reactions involves understanding the relationships between reactants and products. To approach these problems effectively, it’s essential to use a logical, step-by-step method that ensures accurate balancing, correct stoichiometric calculations, and the proper interpretation of results.
Here are some strategies to help tackle such problems:
- Write the Unbalanced Equation – Begin by writing the correct skeletal equation with all the reactants and products. Ensure that all chemical formulas are accurate and represent the substances involved.
- Balance Elements One at a Time – Start with elements that appear in only one reactant and one product, adjusting the coefficients to balance these atoms. Continue to balance the remaining elements until the entire equation is balanced.
- Balance Oxygen and Hydrogen Last – If the equation involves compounds containing oxygen or hydrogen, balance these elements towards the end to avoid complicating the process.
- Use the Correct Coefficients – Ensure that you are using whole numbers for coefficients. If necessary, multiply through by a common factor to clear any fractions.
- Check the Balance – Once the equation seems balanced, verify that the number of atoms of each element is the same on both sides. Recheck the stoichiometry and ensure that all coefficients are correct.
For example, consider the following reaction:
C₄H₁₀ + O₂ → CO₂ + H₂O
First, balance the carbon atoms by placing a coefficient of 4 in front of CO₂. Then, balance the hydrogen atoms by placing a coefficient of 5 in front of H₂O. Finally, balance the oxygen atoms by adjusting the coefficient of O₂. The resulting balanced equation is:
2 C₄H₁₀ + 13 O₂ → 8 CO₂ + 10 H₂O
By applying these strategies systematically, you can solve chemical reactions accurately and efficiently. With practice, balancing equations becomes a straightforward task that enhances your problem-solving skills in various chemical contexts.
Tips for Answering Multiple Choice Questions
Multiple-choice problems can be challenging, but with the right approach, you can maximize your chances of selecting the correct option. These types of questions often require careful reading, logical deduction, and a methodical strategy to identify the best answer among the choices provided.
Understand the Question Fully
The first step in tackling multiple-choice problems is to read the entire question carefully. Pay attention to any keywords or phrases that can guide your answer. Avoid rushing through the question; ensure that you understand exactly what is being asked before considering the options.
Eliminate Clearly Wrong Answers
Once you’ve read the question, begin by eliminating the options that are obviously incorrect. This narrows down your choices and increases the probability of selecting the correct one. Even if you’re unsure about the correct answer, ruling out a couple of options gives you a better chance of guessing the right one.
For example, if a question asks about a specific process or concept and one of the choices is entirely unrelated, you can confidently discard that option. Similarly, if an answer is too extreme or doesn’t align with what you know, it’s often a good idea to exclude it from your considerations.
By applying these techniques–careful reading, thoughtful elimination, and reasoning–you can effectively navigate multiple-choice problems with greater accuracy and confidence.
Mastering Problem-Solving for Lab Questions
Solving practical problems in laboratory settings requires a blend of theoretical knowledge and hands-on experience. To approach these challenges effectively, it’s essential to follow a systematic strategy that ensures accuracy and efficiency while working with various substances and equipment.
Steps for Effective Problem Solving
Here are key steps to follow when tackling lab-based problems:
- Understand the Objective – Clearly define what the task or problem is asking you to accomplish. This could involve identifying a substance, measuring a reaction, or calculating a specific property.
- Review Relevant Concepts – Before jumping into the practical work, make sure you’re familiar with the principles and methods needed to solve the problem. Refresh your knowledge on any calculations, formulas, or techniques involved.
- Plan Your Approach – Organize your workflow by outlining the steps you’ll need to take. Ensure you have all the necessary tools and reagents, and think through the order in which to carry out the procedures.
- Record Observations – While performing the experiment, carefully note any relevant observations. These may include changes in color, temperature, or any unexpected outcomes that may provide clues to solving the problem.
- Analyze the Results – Once you’ve gathered your data, take time to analyze it. Compare the observed results to theoretical expectations, identify patterns, and draw conclusions based on the data you’ve collected.
Common Pitfalls to Avoid

- Skipping Steps – Always follow the procedure step-by-step. Missing even small details can lead to inaccurate results or missed insights.
- Overlooking Errors – Be diligent about checking for possible errors during the experiment. Recheck measurements, calculations, and equipment setup to minimize mistakes.
- Ignoring Safety Protocols – Never underestimate the importance of safety in the lab. Proper handling of substances and awareness of potential hazards is critical to ensuring both your success and safety.
By following these strategies, you can approach lab problems with confidence and increase your ability to solve complex tasks effectively. With practice and a methodical mindset, mastering problem-solving in the lab becomes a valuable skill in any scientific discipline.
Time Management During Chemistry Exams
Effective time management is crucial when faced with time-limited assessments, especially when dealing with complex topics. The ability to allocate appropriate time to each section or problem ensures that you can complete the task with a clear and organized approach. Without proper planning, it’s easy to spend too much time on a single question and risk not finishing the entire test.
Start by Prioritizing – As soon as you receive the test, take a few moments to quickly scan through the entire paper. Identify which sections are the most challenging and which ones you are confident about. Begin with the easier sections to build confidence and save the harder problems for later when your mental energy is at its peak.
Allocate Time Wisely – Divide the total time available by the number of sections or questions. Assign a specific amount of time to each task based on its complexity. For example, if certain sections require longer calculations, allow extra time for those tasks. Use a watch or timer to stay on track, and keep an eye on the clock as you progress.
Stay Flexible – While it’s important to stick to your time plan, sometimes unexpected difficulties may arise. If you’re stuck on a particular question, move on to the next one and come back to it later. This will help you avoid wasting time on a single problem that may not be worth as much as others.
Double-Check Your Work – If time permits, allocate the last few minutes to review your answers. Check for any calculation errors, missed units, or incomplete responses. This final review can make a significant difference in the accuracy of your submission.
With effective time management strategies, you can maximize your efficiency, reduce stress, and increase the likelihood of completing all tasks successfully within the allotted time.
Understanding Calibration and Standardization
Accurate measurements are vital in any scientific process, especially when precise results are necessary for testing and analysis. Calibration and standardization are essential techniques used to ensure the reliability of instruments and methods. These procedures ensure that the tools used for measuring substances or reactions are providing correct and reproducible data.
Calibration refers to the process of adjusting an instrument to match known reference points or standards. This helps correct any discrepancies in the measurement readings. Without calibration, instruments may provide inaccurate data, which can lead to faulty conclusions. It is important to regularly calibrate instruments, especially when they are used for precise measurements.
- Establishing Reference Points: Using known standards or certified reference materials to compare and adjust instrument readings.
- Adjusting the Instrument: Tweaking the instrument settings to match these standards, ensuring accuracy in future measurements.
- Verifying Performance: After calibration, it’s crucial to test the instrument’s performance to ensure it’s within the acceptable range.
Standardization is the process of preparing a solution with a known concentration. This allows one to use that solution to measure unknown substances. Standardizing solutions is particularly important when performing titrations or other quantitative analyses. By knowing the exact concentration of a solution, it is easier to compare it with other substances and achieve accurate results.
- Using Primary Standards: These are highly pure substances that are used to prepare solutions with known concentrations.
- Performing Titrations: Standardized solutions are often used to titrate unknown substances, allowing for accurate concentration determination.
- Ensuring Consistency: Standardization is an ongoing process to ensure that solutions remain accurate and reliable over time.
Both calibration and standardization are critical components in ensuring the precision of scientific measurements. They help eliminate errors, reduce variability, and provide confidence in the results obtained during testing or analysis.
How to Write Effective Lab Reports
Writing clear and concise lab reports is a crucial skill for documenting experimental results. A well-organized report not only helps in presenting findings but also allows others to understand and replicate the work. To produce an effective report, it is essential to communicate both the process and the outcomes in a structured manner.
Start by outlining the purpose of the experiment, ensuring that the objective is clearly stated. The introduction should briefly explain the theory behind the experiment, setting the stage for the methodology. Be precise in detailing the materials and methods used so that others can recreate the experiment accurately. This section should describe each step taken, including any instruments or techniques used, along with any calculations made during the process.
The results section should present the data collected in a clear format, often using tables, charts, or graphs to enhance readability. Avoid unnecessary interpretations here; the goal is to showcase raw data for the reader’s analysis. In the discussion, you can interpret the findings, compare them to expected outcomes, and explain any discrepancies. This section allows you to highlight the significance of your results and explore possible reasons for any anomalies encountered.
- Title: Choose a clear, descriptive title for your report.
- Introduction: Define the purpose of the experiment and provide background information.
- Materials and Methods: Detail the equipment and procedures used during the experiment.
- Results: Present data in a logical and organized manner using tables and graphs.
- Discussion: Interpret the data and draw conclusions based on your findings.
- Conclusion: Summarize the main points, including the experiment’s success or areas for improvement.
Finally, ensure that your report is properly formatted and free from grammatical errors. Clear, concise writing is key to making your findings easily accessible and understandable. A well-written report demonstrates your understanding of the experiment and the ability to communicate scientific information effectively.
Reviewing Past Exam Papers for Success
One of the most effective strategies for preparing for an assessment is reviewing previous tests. By examining past materials, you gain valuable insight into the types of topics that are often covered and how questions are typically structured. This approach allows you to identify patterns in the content, familiarize yourself with the format, and refine your problem-solving skills, making it an essential part of the preparation process.
Start by gathering a variety of past papers, ensuring you have a broad selection that represents different topics and difficulty levels. As you work through each document, focus on understanding the reasoning behind the correct answers. This can help you grasp the key concepts being tested, as well as the techniques used to solve particular problems. Take note of any recurring themes or questions that seem to appear across multiple assessments–this could indicate core topics that are highly important for your studies.
- Analyze the format: Familiarize yourself with the question formats (e.g., multiple choice, short answer, long answer).
- Identify patterns: Look for recurring themes or frequently tested topics to prioritize during your study sessions.
- Time management practice: Simulate the test conditions by timing yourself while solving past papers.
- Review incorrect responses: Understand why certain answers were wrong and learn the correct approach for solving similar problems.
- Seek feedback: If possible, ask a teacher or peer to review your solutions and provide constructive criticism.
Finally, consider practicing under timed conditions to simulate the pressure of the actual assessment. This exercise not only improves your time management but also boosts your confidence. By reviewing past materials, you prepare yourself to tackle a wide range of possible questions and ensure a deeper understanding of the subject matter. This method of preparation can make all the difference when it comes to achieving success in your assessments.
Building Confidence for Analytical Chemistry Exams
Confidence is a crucial factor when preparing for any assessment. The more prepared you feel, the less stress you’ll experience on the day of the test. Achieving this confidence comes from consistent preparation, practicing key concepts, and adopting effective study techniques. By breaking down complex topics into manageable sections and regularly reviewing your progress, you can build a solid foundation of knowledge and reduce any anxiety related to assessments.
Preparation Techniques to Build Confidence
There are several effective strategies you can use to gain confidence as you approach your tests. The key is consistency and ensuring you cover all important areas without rushing. Here are some useful tips:
- Regular Review: Revisit topics you find difficult to understand until they become clearer. Revising periodically prevents last-minute cramming and reinforces knowledge retention.
- Active Learning: Engage in active study methods such as solving practice problems, conducting mock assessments, or teaching concepts to others. This reinforces understanding and boosts confidence.
- Break Tasks into Chunks: Divide your study sessions into shorter, focused blocks of time, concentrating on specific topics. This makes learning less overwhelming and more manageable.
- Group Study: Join or form a study group with peers who can provide feedback and share different perspectives. This allows you to identify gaps in your understanding and learn from others.
Strategies for Test Day
Once you’ve prepared thoroughly, the next step is to ensure that your confidence shines through on the day of the test. Consider the following strategies to maximize your performance:
- Positive Visualization: Take a few moments before the test to mentally visualize yourself answering questions confidently and successfully.
- Stay Calm: Managing stress is essential. Take deep breaths, relax, and approach each question with a calm mindset.
- Time Management: Make sure you allocate time to each section and avoid spending too much time on any one question. Practice time management in mock tests to ensure you’re prepared for the real assessment.
Track Your Progress
As you move forward with your studies, keep track of your progress by identifying areas of improvement. Here’s a simple table to help you monitor your growth and areas that need more attention:
| Topic | Confidence Level | Areas to Improve |
|---|---|---|
| Concept 1 | High | None |
| Concept 2 | Medium | Practice more problems |
| Concept 3 | Low | Review theory |
By consistently tracking your progress and staying focused on the areas that need improvement, you will gain confidence, reducing test anxiety and improving your overall performance. The combination of preparation, practice, and positive thinking will help you tackle assessments with a greater sense of assurance.