Atomic Theory Exam Answers for Success

Understanding the fundamental principles of matter is essential for anyone studying the building blocks of the universe. Whether you’re preparing for a test or seeking to strengthen your knowledge, grasping these foundational ideas can make a significant difference in your performance. The key to mastering these concepts lies in understanding the roles of particles, their interactions, and how they form the structure of everything around us.
In this section, we will explore how different components of matter are categorized and how they interact. By breaking down complex ideas into simpler elements, we can gain clarity and build a strong foundation for further studies. From basic structures to advanced principles, knowing these core concepts is crucial for navigating any related questions that may arise.
Focus on understanding rather than memorization. The goal is not just to recall facts, but to truly comprehend how these principles work together to shape the world at its most fundamental level. With a clear grasp of these concepts, you’ll be well-equipped to address any challenge that comes your way.
Understanding Key Concepts of Matter
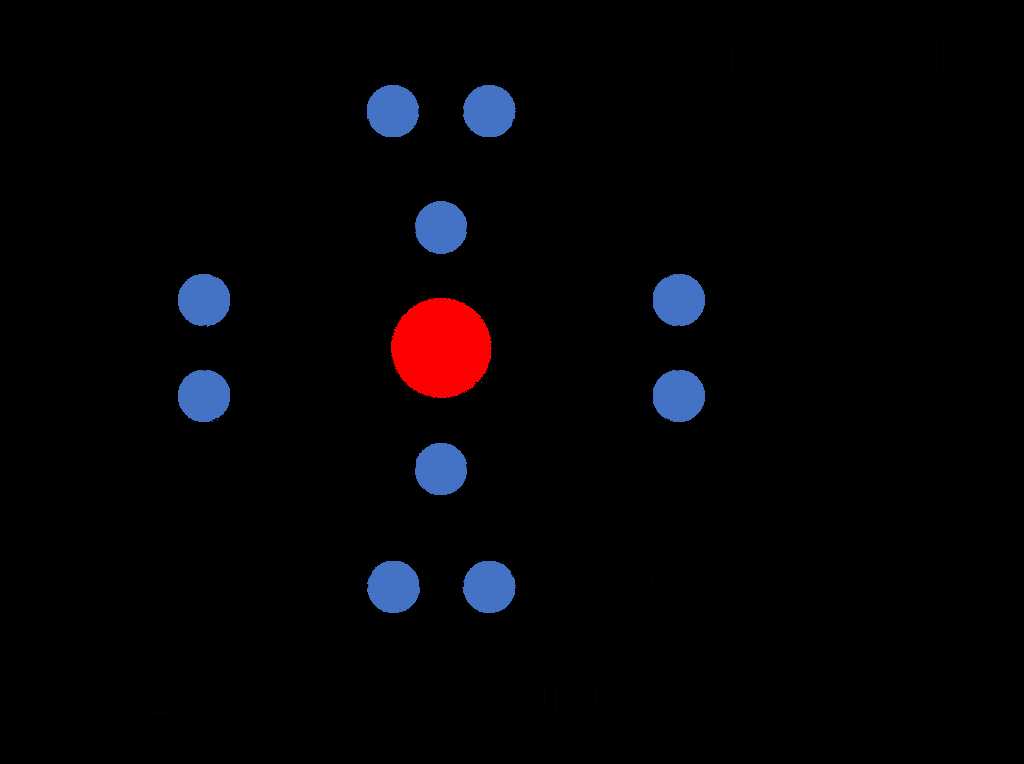
When approaching questions related to the structure of matter, it’s important to recognize the essential components and how they interact. This section provides insights into the key elements that make up all substances and how to effectively address queries related to their properties and behavior. By grasping the core ideas, you can confidently tackle challenges that involve the fundamental principles of matter.
Here are some vital concepts to focus on when preparing for assessments related to matter’s building blocks:
- Basic Particles: Understanding protons, neutrons, and electrons and their roles in forming atoms.
- Particle Arrangement: How different particles are arranged within an atom and how this affects its properties.
- Electron Behavior: The movement of electrons and its impact on chemical reactions and bonding.
- Energy Levels: The concept of energy levels or shells in which electrons reside and their importance in determining chemical characteristics.
- Periodic Table Patterns: Recognizing patterns in elements based on their atomic structure and how these patterns relate to their chemical behavior.
Effective preparation requires an understanding of how these basic principles can be applied to real-world situations. Here are some tips for addressing questions related to these topics:
- Familiarize yourself with the historical development of particle models and how they evolved over time.
- Practice identifying elements based on their properties, such as atomic mass and number.
- Study how different atomic structures influence the formation of compounds and molecules.
- Review key equations and concepts that relate to particle interaction and energy states.
With a solid understanding of these principles, you will be better equipped to interpret and respond to a wide range of related questions effectively.
Understanding Atomic Structure Basics
At the heart of all matter lies a basic unit, composed of smaller particles that determine its properties and behavior. Grasping the structure of these units is fundamental to understanding the physical world. The way these particles are arranged and how they interact is essential in forming substances and chemical reactions. This section will explore the basic components of matter and how they are organized at the most fundamental level.
The primary building blocks of matter are protons, neutrons, and electrons. Each plays a specific role in defining the characteristics of an element. The protons and neutrons are located in the core of the unit, while electrons orbit around it. Understanding the properties of each particle is key to comprehending how matter behaves in different environments.
| Particle | Charge | Location | Mass |
|---|---|---|---|
| Proton | Positive | Nucleus | 1 amu |
| Neutron | Neutral | Nucleus | 1 amu |
| Electron | Negative | Orbitals | 0 amu |
Each of these particles has a specific role in defining the identity and behavior of substances. The number of protons in the core determines the type of element, while the electrons influence how the element interacts with others. By understanding these basics, one can start to make sense of how complex compounds and materials are formed.
Key Concepts in Atomic Models
To fully understand the behavior of matter, it’s essential to explore how the fundamental units of matter are structured. Over time, different models have been proposed to explain the arrangement and behavior of the smallest particles that make up everything around us. Each model has its own unique perspective, helping scientists gain insights into the nature of substances and their interactions. Understanding these concepts provides a clearer view of how different materials and reactions work at the most basic level.
The Bohr Model
One of the most significant models in the study of matter is the Bohr model, which introduced the idea of electrons orbiting the nucleus in fixed paths or shells. This concept simplified the way scientists understood energy levels and how electrons can jump between them when they gain or lose energy. While this model has been refined over time, it remains fundamental in understanding the behavior of many elements.
Modern Quantum Model
As research advanced, the Bohr model gave way to more complex quantum models. These modern theories propose that electrons do not follow defined paths but instead exist within certain probabilities in regions around the nucleus. This model accounts for the wave-like behavior of particles and provides a deeper understanding of the relationships between energy, location, and matter’s behavior.
Exploring Atomic Number and Mass
The identity of an element and its characteristics are primarily determined by two key properties: the number of particles in its core and the total mass of the unit. These fundamental aspects not only define the element but also influence its chemical behavior and interactions with other elements. In this section, we will examine how these properties are determined and why they are essential in understanding the structure of matter.
Understanding Atomic Number
The number of protons in the central core of an atom is known as its atomic number. This value is crucial because it defines the element itself–each element has a unique number of protons. For example, an element with one proton is hydrogen, while an element with six protons is carbon. The atomic number also helps to determine the element’s position on the periodic table, providing insight into its properties and reactivity.
Understanding Atomic Mass
Mass, on the other hand, is the combined weight of protons and neutrons within the atom’s core. This total mass is typically measured in atomic mass units (amu) and varies slightly depending on the number of neutrons present in the nucleus, which results in different isotopes of the same element. The mass of an element influences its density, stability, and behavior in reactions, making it an essential factor in understanding material properties.
Electron Configuration and Periodicity
The arrangement of electrons within an element plays a crucial role in determining its chemical properties and behavior. This organization follows specific rules and patterns that are essential for understanding how elements interact with one another. The periodicity, or repeating trends, in the properties of elements can be directly linked to how electrons are distributed across different energy levels. By examining these configurations, one can predict the reactivity and bonding tendencies of elements.
Electron configuration refers to the way electrons are arranged in the various energy levels or shells surrounding the nucleus. These arrangements follow specific rules, such as the Pauli exclusion principle and Hund’s rule, which help determine the stability of an atom. Elements in the same group on the periodic table share similar electron configurations, which explains their similar chemical properties.
The concept of periodicity describes the recurring patterns of properties as you move across or down the periodic table. For example, elements with similar electron configurations in the same column exhibit similar reactivity and bonding characteristics. Understanding these patterns helps to predict how elements will behave in chemical reactions, making the study of electron configuration an essential aspect of chemistry.
Quantum Theory in Atomic Chemistry
The behavior of particles at the smallest scales can be vastly different from what we observe in our everyday experience. In the realm of matter’s fundamental components, classical mechanics often falls short of explaining phenomena such as energy levels and electron behavior. Quantum mechanics, a more advanced framework, provides the tools necessary to describe the behavior of particles within an atom, offering insights into their movement, energy states, and interactions. This understanding is essential for advancing chemistry, particularly when it comes to explaining the structure and reactivity of elements.
Wave-Particle Duality

One of the core principles of quantum mechanics is wave-particle duality, which suggests that particles like electrons exhibit both wave-like and particle-like properties. This dual behavior helps explain phenomena that cannot be described by classical physics, such as interference and diffraction patterns. Electrons, for instance, can be thought of as existing in “clouds” of probability, rather than following specific paths around the nucleus. This concept is central to understanding how atoms and molecules absorb and emit energy, as well as how they bond with other elements.
Energy Quantization

Another key aspect of quantum mechanics in the context of matter is energy quantization. Electrons within an atom occupy discrete energy levels, and they can only gain or lose energy in specific amounts. This explains why atoms can only absorb or emit light at certain wavelengths, a phenomenon that is fundamental to understanding atomic spectra. The quantization of energy also impacts the stability of atoms and their reactivity, as electrons must jump between these energy levels in a way that influences how atoms interact with one another during chemical reactions.
Atomic Theory and Chemical Bonds
The way in which atoms interact and form connections is fundamental to understanding the formation of substances and their properties. These connections, or bonds, are formed when atoms share or transfer electrons, allowing them to achieve a more stable state. Understanding the underlying principles behind these bonds helps explain the variety of substances that can be created, from simple molecules to complex compounds. This section will explore the different types of interactions between atoms and how they lead to the creation of new materials.
Covalent Bonds
A covalent bond is formed when two atoms share electrons in order to achieve a more stable electron configuration. This type of bond typically occurs between non-metal atoms, where the atoms work together to complete their outer electron shells. The sharing of electrons allows the atoms to form a stable arrangement, often resulting in the creation of molecules. The strength of covalent bonds is influenced by the number of electrons shared and the specific arrangement of atoms within the molecule.
Ionic Bonds
In contrast to covalent bonds, ionic bonds occur when one atom transfers electrons to another, leading to the formation of charged particles, or ions. One atom becomes positively charged (cation) and the other becomes negatively charged (anion). These opposite charges attract, holding the atoms together in a stable bond. Ionic bonds are typically formed between metal and non-metal atoms and are responsible for the properties of many salts and minerals, including their high melting points and electrical conductivity when dissolved in water.
Differences Between Isotopes Explained
Atoms of the same element can exist in different forms, where they share the same number of protons but differ in their number of neutrons. This variation in neutron number leads to differences in mass and, in some cases, stability. While these forms still belong to the same element, they can exhibit unique properties and behaviors. Understanding the differences between these variations helps explain phenomena such as radioactivity and the diverse applications of certain elements in fields like medicine and energy.
The main distinction between isotopes of an element lies in their mass. Isotopes have the same chemical properties because they have the same number of electrons and protons, which govern the element’s chemical behavior. However, the difference in the number of neutrons leads to variations in their atomic mass. Some isotopes are stable, while others are unstable and decay over time, emitting radiation. This radioactive decay is a fundamental aspect of many scientific processes and practical uses, from dating ancient materials to treating cancer.
Historical Evolution of Atomic Theory
The understanding of matter has evolved significantly over time, with early ideas about the composition of substances gradually giving way to more precise and detailed models. Initially, philosophers speculated about the fundamental building blocks of the universe, but it wasn’t until centuries later that experimental evidence began to shape our modern understanding. The journey from ancient concepts to contemporary models has been marked by key discoveries that transformed how we view the fundamental nature of matter.
Early Philosophical Ideas
The origins of the idea of indivisible particles can be traced back to ancient civilizations. The earliest recorded thoughts came from Greek philosophers, who speculated that all matter was made up of tiny, indestructible particles. These ideas, although not based on experimental evidence, laid the groundwork for future scientific developments. Key figures include:
- Democritus: Proposed that everything is made of tiny, indivisible particles called “atomos.”
- Leucippus: Collaborated with Democritus on the concept of particles making up all matter.
The Scientific Revolution
Fast forward to the 17th and 18th centuries, where scientific experimentation began to challenge and expand earlier ideas. New discoveries, particularly in the fields of chemistry and physics, helped define a more concrete model of matter. Some of the most pivotal moments include:
- John Dalton: Introduced the first modern model of the atom in the early 19th century, suggesting that elements are made of indivisible atoms that combine in fixed ratios.
- J.J. Thomson: Discovered the electron in 1897, showing that atoms are divisible and contain smaller particles.
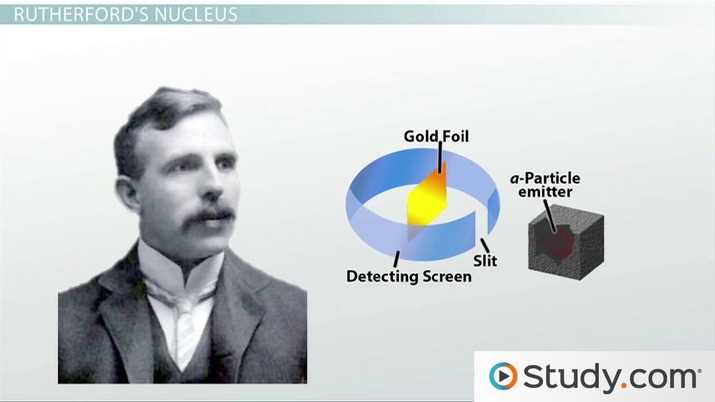
- Ernest Rutherford: Conducted the gold foil experiment in 1909, revealing the existence of a small, dense nucleus at the center of the atom.
Importance of Valence Electrons in Chemistry
The outermost electrons in an atom play a crucial role in determining how that atom interacts with others. These electrons are involved in the formation of bonds, which are the basis for the creation of compounds and materials. Understanding the behavior of these outer electrons is essential for explaining chemical reactions, the properties of substances, and their ability to combine in different ways. In this section, we explore the significance of these electrons and their role in the structure and reactivity of elements.
Valence electrons are responsible for the chemical properties of an element. They govern how atoms bond with one another, whether through the sharing of electrons or the transfer of charge. The number of valence electrons in an atom dictates its reactivity, stability, and the types of chemical bonds it can form. The arrangement of these electrons influences the overall behavior of elements in a wide range of reactions, from simple to complex. Below are some key points about the importance of valence electrons:
- Chemical Bond Formation: Valence electrons are directly involved in the formation of both covalent and ionic bonds.
- Reactivity: Elements with similar numbers of valence electrons tend to have similar chemical behaviors, leading to the classification of elements into groups on the periodic table.
- Periodic Trends: The number of valence electrons influences periodic trends such as electronegativity, ionization energy, and electron affinity.
For example, elements in the same group of the periodic table share the same number of valence electrons, which is why they exhibit similar chemical properties. Understanding these trends is fundamental for predicting how elements will react in different conditions, making valence electrons a critical concept in chemistry.
Understanding Atomic Spectra and Emission
The way elements absorb and emit energy can reveal important details about their structure and behavior. When atoms interact with energy, such as light or heat, they can absorb or release specific wavelengths, resulting in a spectrum unique to each element. This interaction provides valuable insights into the arrangement of electrons and the energy levels within atoms. Understanding these patterns is essential for a deeper knowledge of atomic behavior, the nature of light, and various practical applications in fields like chemistry, physics, and astronomy.
Emission and Absorption Spectra
When atoms are exposed to energy, their electrons are excited to higher energy levels. As the electrons return to their original state, they release energy in the form of light. The emitted light has specific wavelengths, which form the element’s emission spectrum. On the other hand, when atoms absorb light, the specific wavelengths corresponding to the energy required to excite electrons are absorbed, creating an absorption spectrum. These spectra are unique to each element and serve as a “fingerprint” that can be used to identify substances in various applications.
| Element | Emission Spectrum Color | Common Application |
|---|---|---|
| Hydrogen | Red, Blue, Violet | Flame Tests, Spectroscopic Analysis |
| Sodium | Yellow | Street Lights, Flame Tests |
| Neon | Red-Orange | Neon Signs, Spectroscopy |
Practical Applications of Spectra
The study of emission and absorption spectra is not only fundamental to understanding atomic structure, but also has wide-ranging applications. In analytical chemistry, it allows scientists to determine the composition of substances by observing the specific wavelengths of light they emit or absorb. In astronomy, spectra are used to analyze the light from distant stars and galaxies, providing crucial information about their composition, temperature, and motion. Understanding these spectral patterns also aids in the development of technologies such as lasers and fluorescent lights.
Role of Neutrons in Atomic Stability
In the structure of an atom, there are particles that play a crucial role in maintaining its stability. Neutrons, which are neutral particles found within the nucleus, are integral to the overall balance of an atom. While protons contribute to the positive charge of the nucleus and electrons determine chemical behavior, neutrons help to stabilize the nucleus by reducing the repulsive forces between positively charged protons. The number of neutrons in an atom can influence its properties, including its mass and nuclear stability.
Neutrons serve several key functions in ensuring the stability of atoms. Here are the primary roles they play:
- Balancing Nuclear Forces: Neutrons help to offset the electrostatic repulsion between positively charged protons, allowing the nucleus to remain intact and stable.
- Influencing Isotopes: The number of neutrons in an atom determines which isotope it is. Isotopes are variants of the same element that differ in neutron count, and their stability can vary based on the neutron-to-proton ratio.
- Enabling Nuclear Reactions: Neutrons are involved in many types of nuclear reactions, including fission. Their ability to interact with nuclei makes them essential for energy production in nuclear reactors.
However, an imbalance in the number of neutrons relative to protons can lead to instability. Atoms with too many or too few neutrons can undergo radioactive decay as they seek a more stable configuration. This process is essential for understanding both natural radioactivity and the behavior of radioactive materials in various scientific and industrial applications.
Protons and Their Charge in Matter
Within the core of an atom, there are essential particles that define its properties and behavior. Among these, protons are critical in determining the nature of matter. They carry a positive charge, which plays a fundamental role in the formation of the atom’s identity and its interactions with other particles. The number of protons in an atom’s nucleus directly influences the element to which the atom belongs, as well as its chemical properties.
Protons are not only central to the composition of atoms but also affect the behavior of matter in various ways. Here are some of the key points to consider:
- Positive Charge: Protons carry a positive charge, which helps create the electrical field that holds atoms together. The positive charge of protons balances the negative charge of electrons, ensuring that atoms remain neutral overall.
- Defining the Element: The number of protons in the nucleus, known as the atomic number, determines which element an atom is. For example, hydrogen has one proton, while helium has two.
- Influence on Chemical Reactions: The presence of protons in the nucleus affects how atoms bond and interact with other atoms. Chemical reactions often involve the transfer or sharing of electrons, which is influenced by the arrangement of protons within atoms.
- Stability of the Nucleus: The number of protons also plays a role in the stability of an atom’s nucleus. A balance between protons and neutrons is necessary for the nucleus to remain stable and not undergo radioactive decay.
Understanding the role of protons and their positive charge helps to explain the fundamental properties of matter and how atoms interact with each other. Their influence extends beyond chemistry, impacting fields such as physics, energy production, and materials science.
Models of the Atom Through Time
Throughout history, our understanding of the smallest building blocks of matter has evolved significantly. Early concepts of the structure of matter were based on philosophical ideas, but as science progressed, more precise models emerged. These models have been developed to explain the behavior of atoms, each refining the picture of their structure and the forces that govern them. Over time, these evolving models have laid the foundation for much of modern chemistry and physics.
Here are some of the key models of the atom that have shaped scientific thought over the centuries:
| Model | Key Idea | Key Scientist(s) | Time Period |
|---|---|---|---|
| Dalton’s Model | Atoms are indivisible particles, and each element consists of identical atoms. | John Dalton | 1803 |
| Thomson’s Model | Atoms consist of a positively charged “pudding” with negatively charged electrons embedded in it. | J.J. Thomson | 1897 |
| Rutherford’s Model | Atoms have a small, dense nucleus surrounded by mostly empty space with electrons orbiting around it. | Ernest Rutherford | 1911 |
| Bohr’s Model | Electrons orbit the nucleus in specific energy levels or shells. | Niels Bohr | 1913 |
| Quantum Mechanical Model | Electrons exist in probability clouds or orbitals, not fixed paths, and their behavior is governed by quantum mechanics. | Erwin Schrödinger, Werner Heisenberg | 1926 |
Each model has contributed to the current understanding of atomic structure, with later models providing more accurate representations based on experimental evidence. The transition from classical models to quantum mechanics marks the most recent development, providing the most accurate description of electron behavior and the nature of matter itself.
Key Atomic Theory Contributors
Throughout history, many brilliant scientists have played a crucial role in developing the understanding of the fundamental components of matter. Their groundbreaking ideas, experiments, and models have shaped our knowledge of the universe at its most basic level. These individuals have challenged existing beliefs, introduced new concepts, and laid the groundwork for the field of modern chemistry and physics.
Below are some of the key figures who made significant contributions to our understanding of matter’s building blocks:
- Democritus – An ancient Greek philosopher who first proposed the idea of indivisible particles, later known as atoms. He suggested that everything in the universe was made up of tiny, unchangeable units.
- John Dalton – Introduced the concept that each element is made up of unique, indivisible atoms and formulated the first modern atomic theory, which laid the foundation for chemical reactions and the law of multiple proportions.
- J.J. Thomson – Discovered the electron using the cathode ray tube experiment and proposed the “plum pudding” model of the atom, where electrons were embedded in a positively charged “pudding”.
- Ernest Rutherford – Conducted the famous gold foil experiment, which led to the discovery of the nucleus and the proposal of a model with a dense central nucleus surrounded by orbiting electrons.
- Niels Bohr – Developed a model of the atom where electrons travel in fixed orbits around the nucleus, explaining the discrete energy levels and spectral lines of elements.
- Werner Heisenberg – Introduced the uncertainty principle and helped develop the quantum mechanical model of the atom, where electron positions and velocities are inherently uncertain.
- Erwin Schrödinger – Created a mathematical model that describes the wave-like behavior of electrons, leading to the concept of orbitals and the quantum mechanical model of the atom.
Each of these scientists contributed essential pieces to the puzzle of understanding matter’s structure. Their work has formed the cornerstone of modern atomic science and continues to guide research and innovation today.
Interpreting Atomic Theory Exam Questions
Understanding how to approach questions related to the structure and behavior of matter is crucial for success in assessments. These questions often require a deep understanding of the concepts and the ability to apply theoretical knowledge to practical scenarios. Mastering the art of interpreting these types of questions will not only help in exams but also in real-world applications of scientific principles.
Breaking Down Complex Questions
Many questions may present complex scenarios or ask for the analysis of various phenomena involving the building blocks of matter. A useful strategy is to break the question into manageable parts, identifying key terms and concepts. Look for specific keywords such as “structure,” “energy levels,” or “reaction,” as these will direct you to focus on the most relevant ideas.
Understanding Scientific Terms and Concepts
Familiarity with the terminology used in questions is essential. Pay close attention to terms like “neutrons,” “electrons,” and “nucleus,” and understand their role in different contexts. It’s important to know how these components interact and how they can affect the overall behavior of matter. Questions may also involve applying these concepts to real-world examples, so understanding their practical implications is key.
By systematically breaking down questions and understanding the underlying concepts, you can approach complex problems with confidence and clarity. This strategy will allow you to navigate through tricky scenarios and demonstrate a solid grasp of the subject matter.
Tips for Preparing for Atomic Exams
Proper preparation is key to succeeding in assessments related to the structure and behavior of matter. Success lies in not just memorizing facts but also in understanding how the fundamental components of matter interact and apply to different scenarios. A well-organized study approach can make all the difference when tackling complex topics and answering challenging questions.
One of the most effective strategies is to break down the subject into manageable sections. Focus on understanding core concepts such as the behavior of particles, energy transfer, and how matter behaves under different conditions. Ensure that you grasp the principles behind each concept before diving into memorization. This will make it easier to recall relevant information when needed.
Practice is also essential. Work through past questions or examples to familiarize yourself with the format and the types of problems you may face. This not only helps you refine your problem-solving skills but also boosts your confidence. Remember, it’s not just about knowing the material but also about being able to apply it effectively under exam conditions.
Finally, ensure you’re well-rested and mentally prepared on the day of the assessment. Stress and fatigue can hinder your ability to think clearly, so it’s important to manage your time and energy effectively leading up to the test.