Evaporation and Intermolecular Attractions Lab Answers
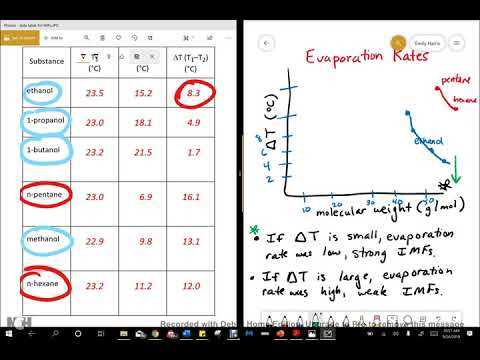
The behavior of liquids undergoing change from a condensed state to a gaseous one involves complex processes driven by molecular dynamics. Various factors influence this transition, including environmental conditions, the properties of the substance, and the forces at play between individual particles. This section delves into the underlying principles that govern how liquids respond to heat and other variables, offering insights into key scientific phenomena.
As molecules move from one phase to another, the interactions between them play a crucial role in determining the rate and efficiency of this process. The role of temperature, pressure, and surface area can all affect how quickly molecules leave the liquid phase. By exploring these concepts in depth, we can better understand not only how substances change state, but also how these transitions are applied in real-world scenarios.
In this guide, we will examine the core principles behind these molecular changes, providing explanations for common observations and experimental outcomes. Whether for academic purposes or practical applications, understanding the intricate balance of forces within substances is essential to comprehending the mechanics of phase transitions.
Evaporation Process Explained in Detail
The transition of a substance from liquid to gas is a fascinating phenomenon that occurs when molecules gain enough energy to break free from the surface of a liquid. This process is influenced by several factors, including temperature, pressure, and the nature of the liquid itself. As the molecules at the surface escape into the air, they leave behind the remaining liquid, which gradually cools as a result of the energy loss.
At the molecular level, the movement of particles plays a key role in this transition. In a liquid, particles are constantly in motion, but they are held together by forces that prevent them from freely dispersing into the surrounding environment. When heat is applied, the molecules at the surface gain enough kinetic energy to overcome these forces, allowing them to escape into the atmosphere as gas molecules. This dynamic process is responsible for various natural occurrences, such as the drying of wet surfaces or the cooling effect felt when sweating.
The rate at which molecules leave the liquid depends on several variables. Higher temperatures provide more energy, speeding up the transition, while increased surface area allows more molecules to escape at once. Similarly, low humidity or pressure can facilitate the process, as the surrounding air can absorb more of the vapor released from the liquid. Understanding these principles can help explain everyday events, from the drying of clothes on a sunny day to the cooling effects of water bodies during hot weather.
Understanding Intermolecular Forces in Liquids
The behavior of liquids is largely dictated by the forces that act between individual molecules. These forces determine how tightly molecules are held together, influencing the physical properties of the substance, such as viscosity, boiling point, and surface tension. In liquids, these forces govern not only how molecules interact with each other but also how they respond to external conditions like temperature or pressure.
There are several types of forces that can exist between molecules, each contributing differently to the overall behavior of the substance. The strength of these forces can vary, with some being relatively weak, while others can be much stronger. The key forces in liquids include:
- Dipole-dipole interactions: These occur between molecules with permanent dipoles, where the positive end of one molecule is attracted to the negative end of another.
- Hydrogen bonding: A special type of dipole-dipole interaction, where a hydrogen atom bonded to a highly electronegative atom (like oxygen or nitrogen) is attracted to another electronegative atom.
- London dispersion forces: These are weak forces that arise from temporary fluctuations in electron distribution, which create momentary dipoles in nonpolar molecules.
Each of these forces plays a vital role in shaping the characteristics of a liquid. For example, substances with strong hydrogen bonds tend to have higher boiling points, as it takes more energy to break these bonds. Conversely, substances with only London dispersion forces may have lower boiling points due to the relative weakness of these interactions. Understanding the different types of molecular forces allows scientists to predict how liquids will behave under various conditions.
These forces also explain why some liquids can form cohesive structures, like drops of water, while others spread out more easily. The balance between attractive and repulsive forces within a liquid can influence everything from surface tension to the rate at which molecules transition between states.
The Role of Temperature in Evaporation
Temperature plays a crucial role in the transition of a liquid to its gaseous form. When the temperature of a substance rises, the energy of its molecules increases, causing them to move faster. This heightened movement can result in a greater number of molecules reaching the surface and escaping into the surrounding environment. As a result, the process occurs more rapidly under higher thermal conditions.
At a microscopic level, as the temperature rises, more molecules gain enough kinetic energy to overcome the forces that hold them together in the liquid state. This leads to a faster release of particles into the air. In contrast, at lower temperatures, the molecules move more slowly, making it less likely for them to break free from the liquid phase.
The relationship between temperature and the rate of transition is directly proportional. The higher the temperature, the faster molecules can escape, increasing the overall rate of change. This is why substances tend to change phase more quickly when exposed to heat. Additionally, temperature influences other properties, such as vapor pressure, which further impacts the overall transition process.
In practical terms, this concept explains everyday occurrences such as the quicker drying of clothes on a hot day or the faster cooling of water when it is heated. By understanding how temperature affects the movement of molecules, we can better predict how liquids will behave under different conditions.
How Molecules Escape During Evaporation
When a liquid transforms into gas, the molecules at the surface experience a crucial change. In a liquid, molecules are constantly in motion, but they are held together by attractive forces. For molecules to break free, they must overcome these forces and gain enough energy to move from the liquid phase into the surrounding air. The process begins with the most energetic molecules at the surface, which have the highest chance of escaping.
As heat is applied to the liquid, it increases the kinetic energy of the molecules. Some molecules, especially those near the surface, gain enough energy to overcome the force of attraction from neighboring particles. Once they reach a sufficient energy threshold, they can break free from the liquid, transitioning into the gas phase. This happens continuously, with molecules constantly entering and leaving the liquid state based on their energy levels.
Not all molecules in a liquid are equally likely to escape. Those with more energy–due to factors like temperature or surface area–are more likely to overcome the forces holding them in place. The process of molecules escaping from the surface occurs without disturbing the rest of the liquid, which means that while some molecules transition to gas, others remain behind in the liquid phase, continuing the cycle.
This phenomenon is what allows a liquid to gradually decrease in volume over time, even in a closed container, as molecules escape from the surface. The more energetic the molecules are, the quicker this transition can occur, which is why liquids exposed to higher temperatures or with greater surface areas will generally lose molecules at a faster rate.
Factors Affecting Evaporation Rates
The speed at which a liquid changes into gas is influenced by a variety of factors, each playing a distinct role in determining how quickly molecules can escape the surface. While some factors increase the rate of this transformation, others slow it down or hinder the process altogether. Understanding these variables is crucial for both scientific studies and practical applications.
Temperature
One of the most significant factors in determining how fast molecules transition from liquid to gas is temperature. As heat is applied to a liquid, the kinetic energy of the molecules increases. This causes the molecules to move faster, making it easier for them to overcome the forces holding them together and escape into the air. The higher the temperature, the more energy the molecules have, leading to a faster transition.
Surface Area
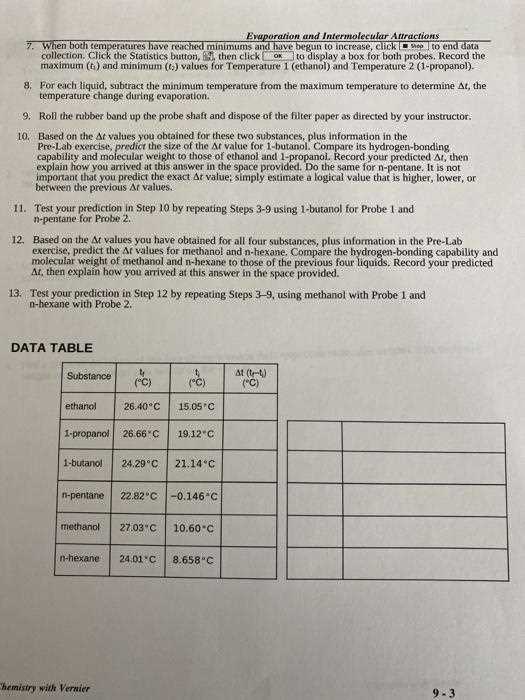
The area exposed to the surrounding environment also affects the rate at which molecules leave the liquid. Larger surface areas allow more molecules to escape at once. This is why a thin layer of liquid will evaporate faster than a deep container of the same substance. The greater the exposure to air, the more molecules can break free from the liquid’s surface.
Other factors, such as air movement, pressure, and humidity, can also significantly impact the rate of molecular transition. For example, lower humidity or higher airflow can accelerate the process by allowing more space for molecules to disperse. Similarly, a lower atmospheric pressure reduces the energy required for molecules to break free, speeding up the transition.
Types of Intermolecular Attractions in Liquids
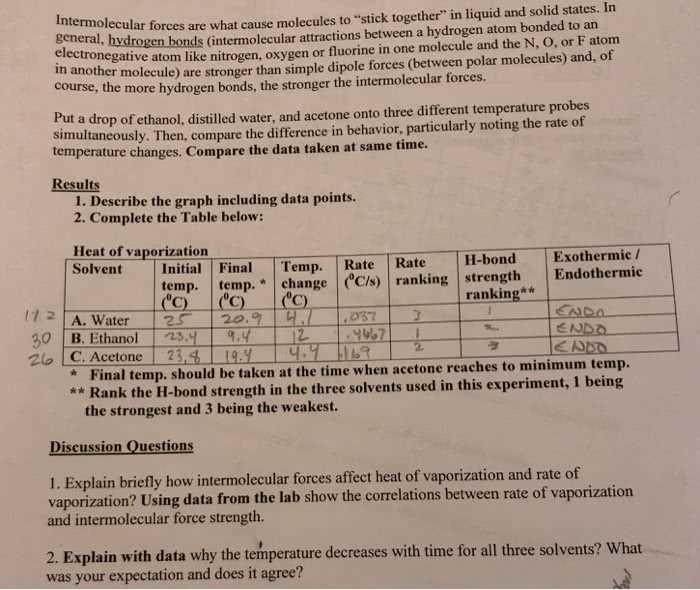
The behavior of liquids is largely determined by the types of forces that act between the molecules. These forces, which vary in strength and nature, influence various properties such as boiling points, viscosity, and surface tension. There are three primary types of attractive forces that govern the interactions between liquid molecules, each contributing differently to the physical characteristics of the substance.
| Type of Force | Description | Example |
|---|---|---|
| Dipole-Dipole Forces | Attractive interactions between molecules with permanent dipoles, where the positive end of one molecule is attracted to the negative end of another. | Hydrochloric acid (HCl), water (H₂O) |
| Hydrogen Bonds | A special case of dipole-dipole interaction where a hydrogen atom attached to a highly electronegative atom (such as oxygen or nitrogen) is attracted to another electronegative atom. | Water (H₂O), ammonia (NH₃) |
| London Dispersion Forces | Weak, temporary forces arising from momentary shifts in electron distribution, creating short-lived dipoles in all molecules, including nonpolar ones. | Oxygen (O₂), methane (CH₄) |
Each of these forces affects how molecules behave in liquids. For example, hydrogen bonds are much stronger than dipole-dipole forces, leading to higher boiling points and increased surface tension. On the other hand, London dispersion forces, though weaker, still play a significant role in nonpolar substances. Understanding these types of forces allows scientists to predict the behavior of different liquids under various conditions.
The Influence of Surface Area on Evaporation
The rate at which a liquid changes into its gas phase is significantly influenced by the amount of exposed surface area. The larger the area through which molecules can escape, the faster the transition occurs. This is because more molecules are in direct contact with the surrounding environment, giving them more opportunities to break free from the liquid’s surface.
When the surface area increases, a greater number of molecules at the liquid’s surface are available to leave. This allows the overall process to proceed more quickly, as more particles are able to gain the necessary energy to escape into the air. Conversely, smaller surface areas limit the number of molecules that can transition, slowing down the process.
Several factors contribute to this relationship:
- Container Shape: The shape of the container holding the liquid affects its exposed surface. For example, a wide, shallow dish allows for more surface area compared to a deep, narrow container.
- Liquid Volume: A larger volume of liquid generally increases the total surface area in contact with the air, though this can also depend on the liquid’s depth and container.
- Spreading of the Liquid: When a liquid spreads out over a surface, such as a puddle on the ground, the exposed area increases, leading to faster phase transition.
This principle is evident in everyday situations. For instance, a spilled drink will typically dry up faster if spread out on a flat surface, as the increased exposure to air accelerates the process. Understanding the connection between surface area and molecular transition helps in various applications, such as drying methods, cooling systems, and chemical reactions.
Why Some Liquids Evaporate Faster
Not all liquids transition to gas at the same rate. The speed at which this process occurs depends on several factors, including the nature of the liquid itself, the temperature, and the surrounding conditions. While some liquids change phase quickly, others do so more slowly, even under identical environmental circumstances. This variation can be attributed to the unique properties of the substances involved.
One major factor is the strength of the forces between the molecules in the liquid. Liquids with weaker forces holding the molecules together generally allow molecules to escape more easily, leading to a faster transition to gas. For example, alcohol tends to evaporate much more quickly than water because the bonds between alcohol molecules are weaker than those in water, allowing the molecules to break free more easily.
Temperature also plays a significant role. A higher temperature provides more energy to the molecules, increasing their speed and making it easier for them to overcome the attractive forces that keep them in the liquid phase. Therefore, liquids exposed to higher temperatures will transition faster than those kept at lower temperatures.
Other factors, such as the surface area of the liquid, the surrounding air pressure, and humidity, also affect the rate at which molecules leave the liquid. For instance, liquids in an environment with low humidity or in a large, shallow container will generally undergo a quicker phase change compared to liquids in a confined space or in more humid conditions.
The Effect of Air Pressure on Evaporation
Air pressure plays a significant role in the transition of liquid molecules into gas. The higher the pressure of the surrounding air, the more difficult it becomes for molecules at the surface to break free and move into the atmosphere. This is because the external pressure exerts a force that counteracts the tendency of molecules to escape the liquid phase, thereby slowing down the process.
Low vs High Air Pressure
At low air pressure, such as at higher altitudes, the resistance to molecules leaving the liquid is reduced. With less atmospheric pressure pressing down on the liquid, molecules can more easily overcome the forces that keep them in the liquid state. This leads to a faster transition from liquid to gas. In contrast, when air pressure is high, such as in lower altitudes or under controlled environments, the process slows down because the higher external pressure creates greater resistance, making it harder for molecules to escape.
Practical Implications of Air Pressure
This relationship between air pressure and the rate at which molecules escape has practical applications in various fields. For example, in cooking, pressure cookers use high air pressure to raise the boiling point of water, which helps food cook faster. On the other hand, the drying of liquids is slower in environments where the air pressure is higher. Similarly, weather patterns, such as the difference in evaporation rates between coastal areas and mountains, are influenced by changes in atmospheric pressure.
Exploring the Evaporation Rate Experiment
The process of liquid molecules transitioning into the gas phase can be studied through controlled experiments. By altering various factors such as temperature, surface area, and air pressure, we can observe how these changes affect the rate at which molecules leave the liquid. The experiment provides valuable insights into the dynamics that govern phase transitions and helps scientists understand the behavior of different substances under various conditions.
Key Variables in the Experiment
To understand how different factors influence the transition from liquid to gas, several variables are manipulated during the experiment. Some of the most important factors include:
- Temperature: Increasing the temperature provides more energy to the molecules, making them move faster and increasing the rate of transition.
- Surface Area: A larger exposed surface area allows more molecules to escape, speeding up the process.
- Air Pressure: Lower air pressure facilitates the movement of molecules into the gas phase, while higher pressure slows it down.
- Humidity: In environments with high humidity, the rate of transition is slower since the air is already saturated with vapor.
Steps Involved in the Experiment
The general procedure for studying the transition rate typically follows these steps:
- Preparation: A specific volume of liquid is placed in a container with a known surface area.
- Control of Variables: The experiment is conducted under controlled conditions, adjusting one factor (e.g., temperature or pressure) at a time while keeping others constant.
- Measurement: The liquid’s volume is measured at regular intervals to determine how much has transitioned to the gas phase.
- Data Collection: Data is recorded for each trial, including changes in temperature, surface area, and air pressure.
- Analysis: The collected data is analyzed to identify patterns and correlations between the variables and the rate of transition.
Through this experiment, researchers can establish clear relationships between various factors and the rate of molecular transition, improving our understanding of this physical process and its real-world applications.
The Relationship Between Evaporation and Boiling
Although both processes involve the transition of molecules from liquid to gas, they occur under different conditions and are driven by distinct mechanisms. While the transition at the surface of the liquid is relatively slow and happens at any temperature, boiling occurs when the liquid reaches a specific temperature at which vaporization happens throughout the entire substance. Understanding the differences between these two processes is key to grasping how liquids behave under various environmental conditions.
Surface vs. Bulk Transition
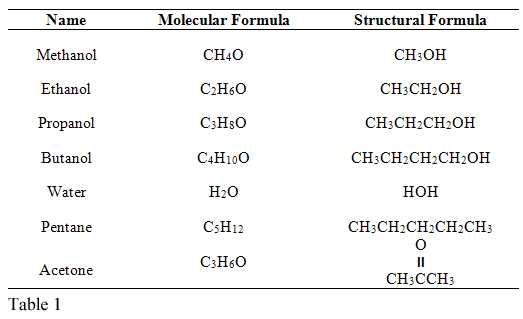
The primary difference between these two processes lies in the location where the change occurs. In the case of boiling, the entire body of liquid reaches a temperature where molecules gain enough energy to escape from both the surface and within the liquid itself. This is in contrast to the gradual transition seen at the surface in the other process, where only molecules at the surface escape into the air.
Temperature Differences
Temperature plays a significant role in both processes. Boiling occurs at a specific temperature known as the boiling point, which depends on external pressure. At this point, the energy of the molecules is high enough to overcome the forces holding them in the liquid phase, allowing for rapid vaporization. On the other hand, molecules in a liquid can escape to the air at any temperature, but the process occurs more slowly at lower temperatures as fewer molecules have enough energy to overcome the attractive forces.
While both processes involve the transition of molecules from liquid to gas, the rate, location, and temperature at which these transitions occur are what distinguish them. Understanding these differences is important for applications ranging from cooking to industrial processes that rely on controlling the rate at which liquids change phase.
Understanding Vapor Pressure and Evaporation
The transition of molecules from a liquid into gas is closely tied to the concept of vapor pressure. This pressure results from the constant movement of molecules in a liquid that collide with the surface of the liquid and the container, creating a measurable force. The relationship between vapor pressure and the rate of molecular transition provides insight into how quickly liquids can change into gas and how environmental factors, such as temperature, can influence this process.
| Factor | Effect on Vapor Pressure |
|---|---|
| Temperature | As temperature increases, the energy of the liquid molecules rises, causing more molecules to escape into the air, leading to a higher vapor pressure. |
| Surface Area | A larger surface area allows more molecules to transition into the air, increasing the rate at which vapor pressure builds. |
| Liquid Type | Liquids with weaker forces between molecules tend to have higher vapor pressures, as their molecules can more easily escape into the gas phase. |
Vapor pressure is a critical factor in determining how quickly molecules leave the liquid phase. The higher the vapor pressure, the faster molecules can transition into the air. This phenomenon explains why volatile liquids, such as alcohol or acetone, change phase more quickly than less volatile substances like water. Temperature is one of the most significant factors that influences vapor pressure–when the liquid is heated, the molecules gain more energy, increasing the number of molecules that can escape and, therefore, raising the vapor pressure.
In summary, vapor pressure is a key factor in the transition of molecules from liquid to gas, influencing both the rate at which molecules escape and the overall phase change process. Understanding this relationship is essential for applications in areas such as chemistry, environmental science, and engineering, where controlling the phase transitions of liquids is necessary.
Impact of Humidity on Evaporation Rates
The amount of moisture already present in the air has a significant influence on how quickly liquid molecules transition into the gas phase. When the air is more saturated with water vapor, the rate at which molecules leave the liquid is slower. This occurs because the air is less capable of holding additional vapor, creating a sort of equilibrium where fewer molecules can escape. On the other hand, in dry conditions, the absence of moisture in the air allows for a faster phase change, as more molecules can move into the atmosphere.
Humidity essentially determines how much room there is in the air for additional molecules to move from the liquid into the gas phase. In environments with high humidity, the air already contains a large amount of vapor, which makes it more difficult for new molecules to escape from the surface. As a result, the liquid tends to stay in its original state for a longer period of time. Conversely, in dry conditions, the low humidity allows more space for molecules to transition, accelerating the process.
In everyday life, we experience this relationship in various ways. For example, clothes dry faster on a hot, dry day compared to a humid one. Similarly, the rate at which puddles of water disappear is much faster in arid climates than in regions with high humidity. Understanding the role of humidity can be useful in a wide range of fields, from meteorology to industrial processes, where controlling moisture levels is essential for achieving desired outcomes.
Evaporation and the Energy Transfer Process
The transition of molecules from liquid to gas is a process driven by energy exchange. In order for molecules to escape from the liquid phase, they need to gain sufficient energy to overcome the attractive forces that hold them in place. This energy is typically absorbed from the surrounding environment, often in the form of heat, and results in a cooling effect on the liquid itself.
During this transition, molecules at the surface of the liquid absorb heat energy, which increases their kinetic energy. When these molecules reach a certain energy level, they are able to break free from the liquid’s surface and enter the gas phase. As these higher-energy molecules leave, the average energy of the remaining liquid decreases, leading to a reduction in temperature.
Several factors influence the rate of energy transfer during this process:
- Temperature: Higher temperatures increase the kinetic energy of molecules, making it easier for them to escape the liquid phase.
- Surface Area: A larger surface area allows more molecules to interact with the surrounding environment, leading to a greater rate of energy absorption and phase transition.
- Humidity: In dry conditions, more heat is available for energy transfer, while in humid conditions, the energy transfer slows due to the already high concentration of vapor in the air.
- Air Movement: Increased airflow can enhance energy transfer by moving heat away from the liquid surface, allowing more molecules to gain enough energy to transition to gas.
This energy transfer is not only important for understanding phase transitions but also for practical applications. For instance, the cooling effect of sweating relies on the energy absorbed as liquid molecules escape into the air, which helps regulate body temperature. In industrial processes, understanding this dynamic is crucial for designing efficient cooling systems and drying techniques.
Common Mistakes in Evaporation Lab Experiments
When conducting experiments that involve the transition of molecules from liquid to gas, several common errors can lead to inaccurate results or misinterpretations. These mistakes can occur at various stages of the experiment, from setup to measurement, and can impact the overall reliability of the findings. Understanding these pitfalls is essential for improving the accuracy and consistency of results in future experiments.
| Mistake | Impact | Prevention |
|---|---|---|
| Inconsistent Temperature Control | Temperature fluctuations can cause variable results, leading to misleading conclusions about the rate of phase change. | Ensure a stable temperature using a calibrated heater or cooling system throughout the experiment. |
| Poor Measurement of Surface Area | Incorrect or inconsistent surface area measurement can affect the rate of transition, causing inaccurate comparisons. | Use precise containers with known and fixed surface areas, or measure the exposed area carefully. |
| Failure to Control Air Flow | Changes in air movement can alter the rate of molecule escape, skewing results. | Conduct the experiment in a controlled environment, ensuring minimal airflow disruption. |
| Ignoring Humidity Variations | Not accounting for the surrounding moisture level can lead to inaccurate conclusions about transition rates. | Monitor and record the humidity levels during the experiment, and maintain consistency between trials. |
| Not Monitoring Initial Liquid Volume | Failure to accurately measure the starting volume of liquid can result in misleading data when calculating rates of change. | Accurately measure and document the initial volume of liquid before starting each trial. |
By being mindful of these common errors, researchers can improve the reliability and accuracy of their experiments. Proper preparation, consistent monitoring of key variables, and attention to detail can help ensure that results reflect the true behavior of liquids under varying conditions.
Applications of Evaporation in Everyday Life
The process of liquid molecules transitioning to the gas phase plays a significant role in many everyday activities. From cooling systems to natural processes, this phenomenon is constantly at work around us. Understanding how this process functions allows us to harness its effects for various practical applications that improve comfort, efficiency, and even health.
One common use of this natural process is in the cooling of our bodies. When we sweat, moisture from the skin absorbs heat from the body and transforms into vapor, thus lowering body temperature. This cooling mechanism is vital for maintaining thermal balance, especially in hot climates or during physical exertion.
Another widespread application is in drying clothes. After washing, clothes hang on a line or are placed in a dryer where heat facilitates the removal of water molecules from the fabric. The increased temperature accelerates the transition from liquid to gas, helping garments dry faster. In areas with low humidity, this process is more efficient, which is why clothes often dry quickly in dry, warm weather.
In the food industry, this process is essential in techniques like drying fruits and vegetables, which extends their shelf life. By removing excess moisture, harmful bacteria and mold are less likely to develop, allowing for long-term storage without refrigeration. Similar principles are used in the preparation of powdered milk, instant soups, and other dehydrated foods.
Lastly, in industrial applications, the process is used for cooling machinery, regulating temperatures in power plants, and even in air conditioning systems. The controlled phase transition helps remove heat from surfaces and cool environments, making these systems indispensable in modern technology.
These examples highlight the crucial role of the phase transition in many aspects of daily life. Whether for comfort, preservation, or technology, understanding and utilizing this natural process is essential to many of the conveniences we often take for granted.