Chapter 3 Active Reading Guide on Carbon and Molecular Diversity
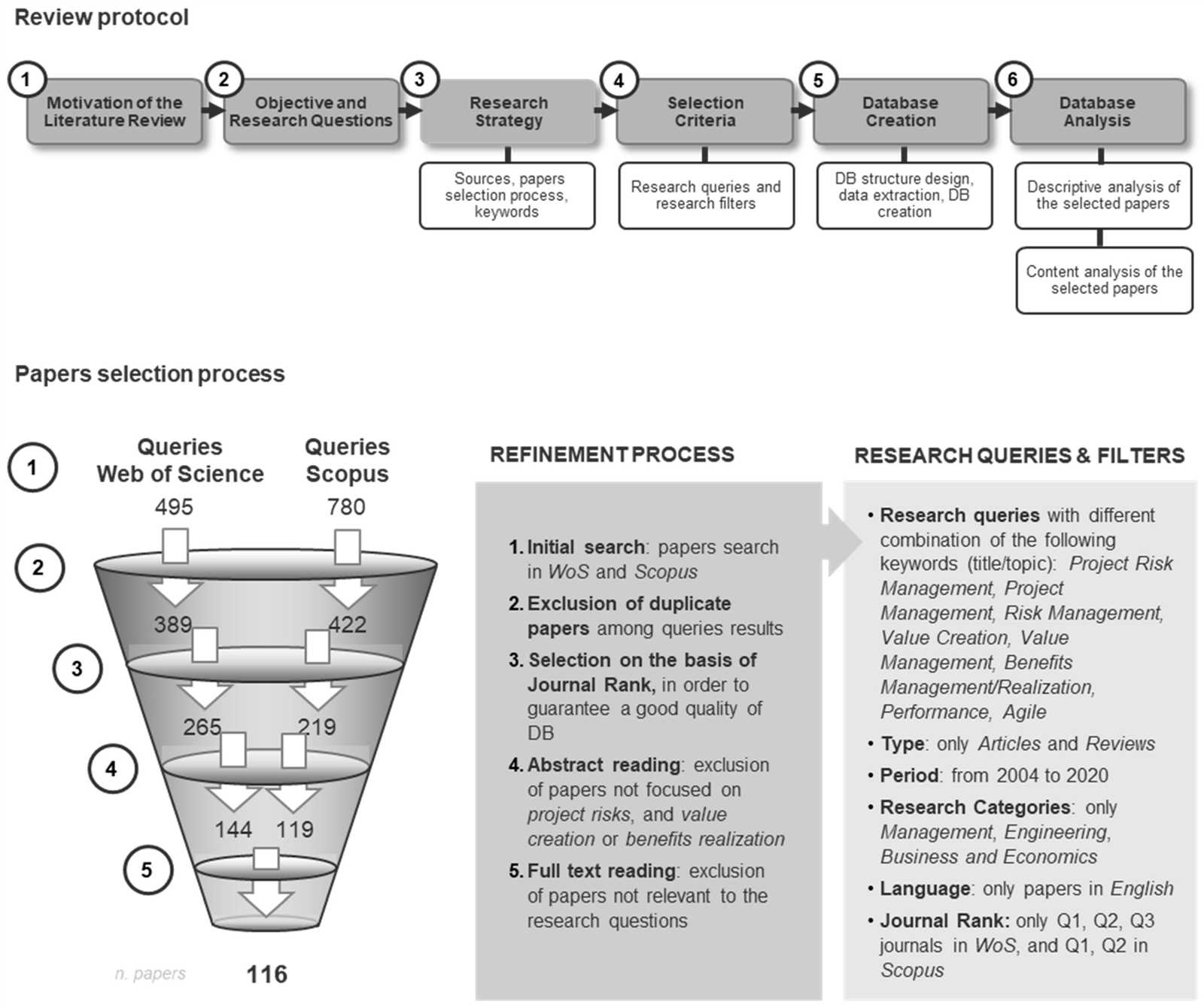
In this section, we explore the foundational principles that shape the structure and function of living organisms. The focus is on understanding the building blocks that make up biological systems and how their unique properties contribute to the vast complexity of life forms. By delving into chemical interactions, we uncover the mechanisms that drive cellular processes and define the characteristics of various biomolecules.
Through a closer examination, you’ll learn how specific atoms and their arrangements create diverse forms of compounds, each playing a crucial role in maintaining the balance within organisms. This knowledge forms the backbone of our understanding of biological chemistry, providing insights into how simple elements combine to support life at the molecular level.
Chapter 3 Active Reading Guide Overview
This section delves into key concepts that underpin the structural complexity of organisms at a chemical level. It emphasizes the essential components that interact within biological systems, revealing how these components form intricate networks to sustain functions critical for life. The focus is on understanding the fundamental principles that govern the formation and function of vital molecules within living organisms.
Key Concepts in Biological Chemistry
The study of chemical elements and their bonds reveals how simple atoms combine to create larger structures that serve various biological roles. These interactions are central to the maintenance of cellular processes and the development of diverse forms of life. By examining these principles, one can gain a deeper appreciation of how life is built from the most basic units of matter.
Understanding Functional Components

Specific elements such as hydrogen, oxygen, nitrogen, and carbon come together to form compounds with distinct properties. These compounds serve as the building blocks for proteins, lipids, carbohydrates, and nucleic acids, each playing a unique role in biological systems. Exploring their structure and function provides essential insights into the chemical foundations of life.
Understanding Carbon’s Role in Life
One element plays a central role in forming the complex structures that are vital to organisms. Its unique bonding properties enable it to create a wide variety of compounds essential for various biological functions. This element’s ability to bond with other atoms allows it to form diverse structures, which are the foundation of numerous critical processes within cells.
Key Features of Carbon
- Versatility in Bonding: It can form strong bonds with many other elements, allowing the creation of diverse molecular structures.
- Formation of Chains and Rings: Its ability to form long chains or rings makes it a key component in many biomolecules.
- Foundation for Complex Compounds: It forms the backbone of essential biological compounds, including proteins, carbohydrates, lipids, and nucleic acids.
Importance of Carbon in Biological Systems
- Energy Storage: Carbon-based compounds such as sugars and fats store energy for cellular activities.
- Structural Integrity: Carbon atoms contribute to the formation of structures that provide stability to cells and tissues.
- Enzyme Function: Many enzymes that drive cellular reactions are built upon carbon-containing molecules.
Through its unique ability to bond and form a variety of structures, this element supports life’s complexity at every level, from individual molecules to entire organisms.
Key Concepts of Molecular Diversity
At the core of biological systems lies a remarkable variety of compounds, each with distinct roles and properties. These substances, which are composed of different atoms arranged in specific ways, are responsible for the complexity of organisms. Their structure directly impacts their function, allowing them to perform a wide range of essential tasks within cells and tissues.
Key Factors Influencing Molecular Variety
| Factor | Description |
|---|---|
| Structure | The way atoms are arranged influences how molecules interact and their role in biological systems. |
| Bonding | The ability of atoms to form different bonds determines the stability and functionality of compounds. |
| Functional Groups | Certain groups of atoms contribute specific properties to molecules, influencing their biological activity. |
These key elements–structural diversity, bonding patterns, and functional group characteristics–allow nature to produce a vast range of compounds with specific functions, contributing to the overall complexity of biological processes.
The Structure of Organic Molecules
Understanding how biological compounds are structured is key to grasping their function within living systems. These compounds, primarily composed of specific elements, have arrangements that determine their behavior and interaction with other molecules. The specific layout of atoms within a compound allows it to carry out essential roles, from energy storage to catalyzing chemical reactions.
Basic Elements in Organic Compounds
- Hydrogen: Forms the backbone of many biological molecules, contributing to their overall stability and structure.
- Oxygen: Essential for energy production and plays a critical role in metabolism and cellular respiration.
- Nitrogen: Found in amino acids and nucleic acids, nitrogen is vital for protein synthesis and genetic information storage.
- Phosphorus: A key element in nucleic acids and energy transfer molecules such as ATP.
Types of Bonds and Their Influence on Structure
- Covalent Bonds: Strong bonds formed when atoms share electrons, ensuring stability in organic molecules.
- Hydrogen Bonds: Weaker than covalent bonds, these help molecules interact with each other and are crucial for protein and DNA structure.
- Ionic Bonds: Formed when atoms transfer electrons, they contribute to the structure of compounds like salts and help maintain cellular function.
By understanding these foundational elements and bonds, we can better appreciate how each compound’s structure supports its specific role in biological systems. The arrangement of atoms, functional groups, and types of bonds ultimately shapes how molecules interact within cells and contribute to life’s processes.
Importance of Functional Groups
Functional groups are critical components in organic compounds, significantly influencing the behavior and properties of molecules. These groups, typically small clusters of atoms, determine how a molecule interacts with other compounds and participate in key chemical reactions. The specific characteristics of functional groups enable molecules to perform various tasks, from energy transfer to acting as catalysts in metabolic pathways.
By altering or adding certain functional groups to a molecule, its overall properties can change dramatically. For example, some functional groups can make a molecule more soluble in water, while others might enhance its reactivity or stability. This adaptability is crucial for the wide range of biochemical processes that sustain life.
Common Functional Groups
- Hydroxyl Group (–OH): Found in alcohols and sugars, it makes molecules more polar, allowing them to dissolve in water.
- Amino Group (–NH2): Present in amino acids, this group plays a key role in protein structure and function.
- Carboxyl Group (–COOH): Essential for the acidity of many compounds, it is found in fatty acids and amino acids.
- Phosphate Group (–PO4): Important in energy transfer and signaling, commonly found in ATP and nucleic acids.
These groups serve as the foundation for the complexity and versatility of organic molecules, enabling them to perform an array of vital functions in biological systems.
Exploring Macromolecules and Their Functions
Macromolecules are large, complex compounds that play essential roles in the structure, function, and regulation of cells. These large molecules are made up of smaller subunits, which are linked together through chemical bonds. Their size and structure enable them to perform a variety of critical tasks, from providing structural support to facilitating chemical reactions within cells. Understanding these compounds is crucial for comprehending how living organisms function on a biochemical level.
Types of Macromolecules

There are four major categories of macromolecules, each serving distinct functions within the body. These include proteins, nucleic acids, lipids, and carbohydrates. Each type of macromolecule has a unique structure that is directly related to its specific role in cellular processes.
- Proteins: Composed of amino acids, they act as enzymes, structural components, and signaling molecules, facilitating nearly all cellular functions.
- Nucleic Acids: DNA and RNA are responsible for storing and transmitting genetic information, guiding the synthesis of proteins and regulating cell activities.
- Lipids: These hydrophobic molecules form membranes and store energy, also acting as signaling molecules and protecting organs.
- Carbohydrates: Sugars and starches serve as energy sources and provide structural support in plants and some animals.
Functions of Macromolecules
Each macromolecule performs specific tasks that are vital to maintaining homeostasis within cells. Proteins, for example, are responsible for catalyzing reactions, building cellular structures, and coordinating cell communication. Nucleic acids carry genetic instructions for protein synthesis and regulate cellular processes. Lipids, due to their hydrophobic nature, form essential barriers such as cell membranes, while carbohydrates supply energy for cellular activities.
By understanding these large molecules and their distinct functions, we gain insight into how organisms maintain order and stability, adapt to their environment, and carry out the processes necessary for survival.
How Carbon Bonds Create Diversity
The ability of one particular element to form a wide variety of structures is central to the complexity of life. This element’s bonding capabilities allow it to form numerous stable connections with other atoms, resulting in a wide array of compounds. These bonds can vary in shape and function, giving rise to the vast diversity of biological molecules found in organisms.
Types of Bonds Formed
- Single Bonds: Two atoms share one pair of electrons, creating simple structures such as methane.
- Double Bonds: Two pairs of electrons are shared, leading to more stable and rigid configurations, as seen in molecules like oxygen.
- Triple Bonds: Three pairs of electrons are shared, allowing even greater stability and typically found in nitrogen compounds.
Impact of Bonding on Structure and Function
- Chain Formation: The ability to form long chains enables the creation of polymers, which are essential for building structures like proteins and DNA.
- Ring Structures: Bonding can also result in ring-shaped molecules, such as glucose, which are important for energy storage.
- Branching: Carbon’s ability to bond in multiple directions creates complex branched structures, enhancing functional diversity in lipids and carbohydrates.
These various types of bonds not only contribute to the vast array of organic compounds but also determine their properties, such as solubility, reactivity, and function. By forming different structures, this element plays a key role in enabling life to thrive in its many forms.
The Role of Isomers in Biology
Isomers are molecules that share the same chemical formula but differ in their arrangement of atoms. These subtle differences in structure can have profound effects on the properties and functions of molecules within living organisms. Isomers can exhibit distinct behaviors despite having identical compositions, which makes them essential in many biological processes. Their ability to form different shapes or orientations enables them to play diverse roles in metabolic pathways and cellular functions.
Types of Isomers
- Structural Isomers: These molecules have the same molecular formula but differ in the connectivity of their atoms, resulting in different structural forms.
- Stereoisomers: While these molecules share the same atomic connectivity, they differ in the spatial arrangement of atoms, affecting how they interact with other molecules.
Biological Significance
- Enzyme Specificity: Isomers can have vastly different effects on enzyme function, as enzymes are often highly specific to the shape of the molecules they interact with.
- Metabolic Pathways: Certain isomers are crucial for various metabolic processes, where even small differences can determine whether a reaction occurs or not.
- Pharmacological Effects: In medicine, isomers may exhibit very different effects on the body, with one isomer being beneficial and another potentially harmful.
Thus, the structural variation among isomers allows organisms to utilize a broad range of compounds, each tailored to specific functions, all of which are essential for the complexity of biological systems.
Building Polymers from Monomers
Polymers are large molecules formed by linking smaller units known as monomers. The process of creating these complex structures is essential for many biological functions, as these macromolecules play critical roles in providing structure, storing energy, and enabling communication within cells. The assembly of monomers into polymers is a vital process that allows for the diversity and complexity of biological systems.
Formation of Polymers
Monomers are connected through chemical reactions that create covalent bonds, resulting in long chains or networks. This process, known as polymerization, can occur in two primary ways: condensation (or dehydration) reactions, where a molecule of water is released, and hydrolysis, where water molecules are added to break bonds. Both methods are fundamental for the synthesis and breakdown of various macromolecules.
- Condensation Reaction: In this process, monomers join together with the elimination of water, forming a covalent bond between them.
- Hydrolysis: This is the reverse reaction, where water is used to break down polymers into their constituent monomers.
Examples of Polymers

- Proteins: Made from amino acids, proteins are polymers that serve as enzymes, structural components, and signaling molecules.
- Nucleic Acids: DNA and RNA are long chains of nucleotides that store genetic information and facilitate protein synthesis.
- Carbohydrates: Polysaccharides like starch and glycogen are formed from simple sugars and function as energy storage molecules.
- Polymers in Lipids: Some lipids, such as triglycerides, consist of smaller fatty acid monomers linked to glycerol molecules.
The ability to form polymers from monomers is a key aspect of cellular metabolism, enabling organisms to create complex structures from simple components. This process contributes to the vast range of biological molecules essential for life functions.
Understanding Hydrolysis and Dehydration
Two fundamental chemical reactions, hydrolysis and dehydration, play a central role in the synthesis and breakdown of complex biological molecules. These processes govern the formation of polymers from simpler monomers and their subsequent disassembly when necessary. Understanding how these reactions work is essential for exploring how living organisms maintain and utilize their biomolecules.
Dehydration Synthesis
Dehydration, also known as condensation, occurs when two smaller molecules are joined together, releasing a molecule of water. This process is vital for building large macromolecules such as proteins, carbohydrates, and nucleic acids. During dehydration synthesis, covalent bonds are formed between monomers, resulting in the creation of larger, more complex structures.
| Step | Reaction |
|---|---|
| 1 | Two monomers come close together. |
| 2 | A molecule of water is removed, creating a bond. |
| 3 | Monomers are linked to form a polymer. |
Hydrolysis
Hydrolysis is the reverse of dehydration. It involves adding water to break down polymers into smaller monomeric units. In this reaction, a water molecule is used to cleave the covalent bonds between the components of a polymer, thus facilitating the breakdown of complex molecules into simpler ones. This process is essential for digestion and energy release in living organisms.
| Step | Reaction |
|---|---|
| 1 | Water is added to a polymer. |
| 2 | The bond between monomers is broken, splitting the polymer. |
| 3 | The result is two or more monomers. |
Both hydrolysis and dehydration are essential for the functioning of biological systems. They enable cells to synthesize the complex molecules needed for structure and function, while also allowing for the breakdown of these molecules when energy or components are required. Understanding these processes reveals much about the dynamic nature of cellular metabolism and energy flow.
Structure and Function of Proteins
Proteins are fundamental components of all living organisms, performing a wide variety of essential tasks within cells. Their structure directly determines their function, with each unique arrangement of amino acids forming a specific three-dimensional shape that allows them to interact with other molecules in highly specialized ways. From providing structural support to catalyzing biochemical reactions, proteins are involved in nearly every aspect of cellular activity.
Proteins are composed of long chains of amino acids that fold into complex shapes. These shapes are crucial to their functions, as they determine how proteins interact with other molecules. The sequence of amino acids in a protein is determined by genetic instructions, and even slight changes in this sequence can have significant effects on its activity.
Levels of Protein Structure
Proteins have four levels of structural organization:
- Primary Structure: The linear sequence of amino acids in a polypeptide chain.
- Secondary Structure: Local folding patterns such as alpha helices and beta-pleated sheets, stabilized by hydrogen bonds.
- Tertiary Structure: The overall three-dimensional shape of a protein, formed by interactions between different parts of the chain.
- Quaternary Structure: The arrangement of multiple polypeptide chains into a functional protein complex.
Functions of Proteins
Proteins serve numerous functions within organisms, including:
- Enzymatic Activity: Proteins act as enzymes, speeding up biochemical reactions essential for cellular processes.
- Structural Support: Structural proteins like collagen provide support and shape to cells and tissues.
- Transport: Proteins like hemoglobin transport molecules such as oxygen throughout the body.
- Signaling: Some proteins act as hormones or receptors, helping cells communicate with one another.
- Immune Defense: Antibodies are proteins that protect the body by identifying and neutralizing foreign invaders.
The unique structure-function relationship of proteins is essential to their roles in biology. Disruptions in protein structure can lead to a wide range of diseases, highlighting the importance of understanding how these molecules function at a molecular level.
Exploring Carbohydrates and Their Uses
Carbohydrates are a vital class of organic compounds that play key roles in providing energy and structural support within living organisms. These molecules are primarily made of carbon, hydrogen, and oxygen, and can range from simple sugars to complex polysaccharides. Their ability to store energy and participate in cellular functions makes them indispensable to life processes.
Depending on their structure, carbohydrates can serve various functions, from being quick sources of energy to forming essential structural components of cells and tissues. The diversity in carbohydrate types allows them to adapt to the specific needs of different organisms and environments.
Types of Carbohydrates
Carbohydrates can be categorized into three main types, each serving different functions within biological systems:
- Monosaccharides: Simple sugars like glucose and fructose that serve as immediate energy sources for cells.
- Disaccharides: Two monosaccharide units linked together, such as sucrose, which is a common energy source for many organisms.
- Polysaccharides: Long chains of monosaccharides that act as storage forms of energy, such as starch in plants and glycogen in animals.
Functions of Carbohydrates
Carbohydrates are critical for various biological functions, including:
- Energy Source: Simple sugars like glucose are broken down in cellular respiration to release energy.
- Energy Storage: Polysaccharides such as starch and glycogen store energy for later use in organisms.
- Structural Support: Carbohydrates like cellulose provide rigidity to plant cells, helping maintain their shape and structure.
- Cell Communication: Carbohydrates on the surface of cells play a key role in cell recognition and signaling, which is important in immune responses and tissue development.
Understanding the structure and functions of carbohydrates highlights their central role in sustaining life. From energy storage to cell signaling, these compounds are integral to the processes that govern biological systems.
Fats and Lipids in Living Organisms
Fats and lipids are essential organic compounds that serve a variety of vital functions in living organisms. These molecules are primarily composed of carbon, hydrogen, and oxygen, but they differ significantly in structure and role. Lipids are hydrophobic, meaning they do not mix easily with water, which allows them to form barriers like cell membranes and store energy efficiently.
Unlike carbohydrates, which are often used quickly for energy, fats and lipids are stored for long-term energy use. They are also involved in insulation, protecting organs, and facilitating the absorption of fat-soluble vitamins. Lipids can vary greatly in structure, from simple triglycerides to more complex phospholipids and sterols, each contributing uniquely to an organism’s biological functions.
Types of Lipids

There are several types of lipids, each serving distinct roles in biological systems:
- Triglycerides: Composed of glycerol and three fatty acid chains, these are the most common form of fat storage in animals and plants. They provide a dense source of energy.
- Phospholipids: These are a key component of cell membranes, forming a barrier that protects and organizes cellular contents.
- Sterols: Such as cholesterol, which are important for cell membrane structure and the synthesis of certain hormones.
Functions of Fats and Lipids
Fats and lipids play several critical roles in maintaining the health and function of living organisms:
- Energy Storage: Lipids are stored in adipose tissue and provide a long-term energy reserve that can be accessed when needed.
- Insulation: Fat deposits act as insulators, helping to regulate body temperature and protect vital organs from external impacts.
- Cell Membrane Formation: Phospholipids create the lipid bilayer of cell membranes, which is essential for cellular integrity and function.
- Hormone Production: Certain lipids are precursors for steroid hormones, which regulate various physiological processes like metabolism and immune function.
In summary, fats and lipids are integral to a wide range of functions in living organisms, from energy storage to cellular structure and signaling. Their unique properties make them indispensable for sustaining biological life and maintaining homeostasis.
The Role of Nucleic Acids in Genetics
Nucleic acids are fundamental to understanding genetic inheritance and the functioning of living organisms. These complex molecules store, transmit, and express genetic information, which dictates the growth, development, and functioning of all cells. They are composed of sequences of nucleotides, which include a sugar, phosphate group, and nitrogenous bases. The specific arrangement of these bases encodes the instructions necessary for producing proteins and regulating cellular activities.
Two main types of nucleic acids–deoxyribonucleic acid (DNA) and ribonucleic acid (RNA)–play distinct but interconnected roles in genetic processes. DNA is primarily responsible for storing genetic information, while RNA plays a crucial role in translating that information into functional proteins.
DNA: The Blueprint of Life
DNA holds the complete genetic blueprint for an organism. It is found in the nucleus of eukaryotic cells and in the cytoplasm of prokaryotes. DNA molecules consist of two long chains of nucleotides twisted into a double helix structure, where the sequence of nitrogenous bases (adenine, thymine, cytosine, and guanine) encodes genetic instructions.
- Genetic Information Storage: DNA stores the instructions for building all the proteins an organism needs to function.
- Replication: DNA can replicate itself, ensuring that genetic material is passed on during cell division.
- Mutation: Changes in the DNA sequence can lead to genetic diversity or contribute to disease.
RNA: The Messenger and Catalyst
RNA serves as an intermediary between DNA and protein synthesis. Unlike DNA, RNA is typically single-stranded and contains ribose sugar instead of deoxyribose. It plays various roles in cells, most notably in protein synthesis, where it helps decode DNA instructions and assemble amino acids into proteins.
- mRNA (Messenger RNA): Carries the genetic message from DNA to the ribosome, where proteins are synthesized.
- tRNA (Transfer RNA): Brings the correct amino acids to the ribosome during protein synthesis.
- rRNA (Ribosomal RNA): Forms a structural component of the ribosome, the site of protein synthesis.
In addition to protein synthesis, RNA can also have regulatory functions in gene expression, influencing how genes are turned on or off in response to environmental signals.
Overall, nucleic acids are the core molecules responsible for transmitting hereditary information and ensuring the proper functioning of living organisms by controlling cellular processes and enabling genetic continuity across generations.
Enzyme Function and Molecular Reactions
Enzymes are essential catalysts that accelerate biochemical processes within living organisms. These protein molecules speed up chemical reactions by lowering the activation energy required for a reaction to take place. Without enzymes, many reactions that are vital for life would occur too slowly to sustain life processes. Each enzyme is specific to a particular reaction or type of reaction, ensuring that the necessary biochemical transformations take place efficiently and accurately within the cell.
Enzymes work by binding to substrates, the reactants in a chemical reaction, forming an enzyme-substrate complex. This interaction facilitates the breaking or forming of bonds, leading to the production of new molecules. After the reaction, the enzyme releases the product(s) and is free to catalyze additional reactions.
How Enzymes Speed Up Reactions
Enzymes enhance the rate of reactions in several ways:
- Lowering Activation Energy: Enzymes reduce the energy required to start a reaction, allowing it to proceed faster.
- Stabilizing Transition States: Enzymes help stabilize the unstable transition state of a reaction, facilitating the conversion of substrates to products.
- Providing an Active Site: The enzyme’s active site is specifically shaped to bind the substrate, ensuring the correct molecules interact at the right time and place.
- Orienting Substrates: Enzymes bring substrates into the correct orientation, increasing the likelihood of a successful reaction.
Factors Influencing Enzyme Activity
Several factors can affect the efficiency and function of enzymes:
- Temperature: Enzymes have an optimal temperature range; excessive heat can denature (unfold) enzymes, making them ineffective.
- pH: Enzymes also have an optimal pH range, outside of which their structure can be altered, affecting their activity.
- Substrate Concentration: As substrate concentration increases, enzyme activity typically increases, up to a point where all enzyme molecules are occupied.
- Inhibitors: Molecules that can decrease enzyme activity by binding to the enzyme, either blocking the active site or altering the enzyme’s structure.
- Activators: Molecules that enhance enzyme activity, often by helping to stabilize the enzyme’s structure or active site.
Understanding enzyme function is crucial for many biological processes, including digestion, metabolism, and cell signaling. Enzymes are involved in breaking down nutrients, synthesizing molecules, and controlling the rate of various biochemical reactions that sustain life. Their specificity, efficiency, and regulation make them indispensable to the complexity of living systems.
The Significance of Chemical Reactions in Life
Chemical processes are at the core of every biological function, driving the transformations that sustain existence. From the breakdown of nutrients to the synthesis of complex molecules, these reactions are essential for maintaining balance within living organisms. Whether producing energy, forming structural components, or facilitating communication within cells, these reactions are responsible for both growth and maintenance in living systems.
In living organisms, reactions occur in tightly regulated sequences to ensure proper functioning. The energy required for these processes comes from chemical bonds, which are broken and formed as molecules react. The efficiency and control of these reactions are critical, as imbalances can disrupt cellular processes and lead to disease or dysfunction.
Types of Chemical Reactions
There are various types of reactions that occur within biological systems, each with its distinct role:
- Exergonic Reactions: These reactions release energy, often used to power cellular activities.
- Endergonic Reactions: These require energy input to proceed, typically driven by the energy produced in exergonic reactions.
- Redox Reactions: In these reactions, electrons are transferred, playing a key role in cellular respiration and energy production.
- Synthesis Reactions: Molecules are built from smaller units, contributing to growth and repair of tissues.
Enzymes as Catalysts in Reactions
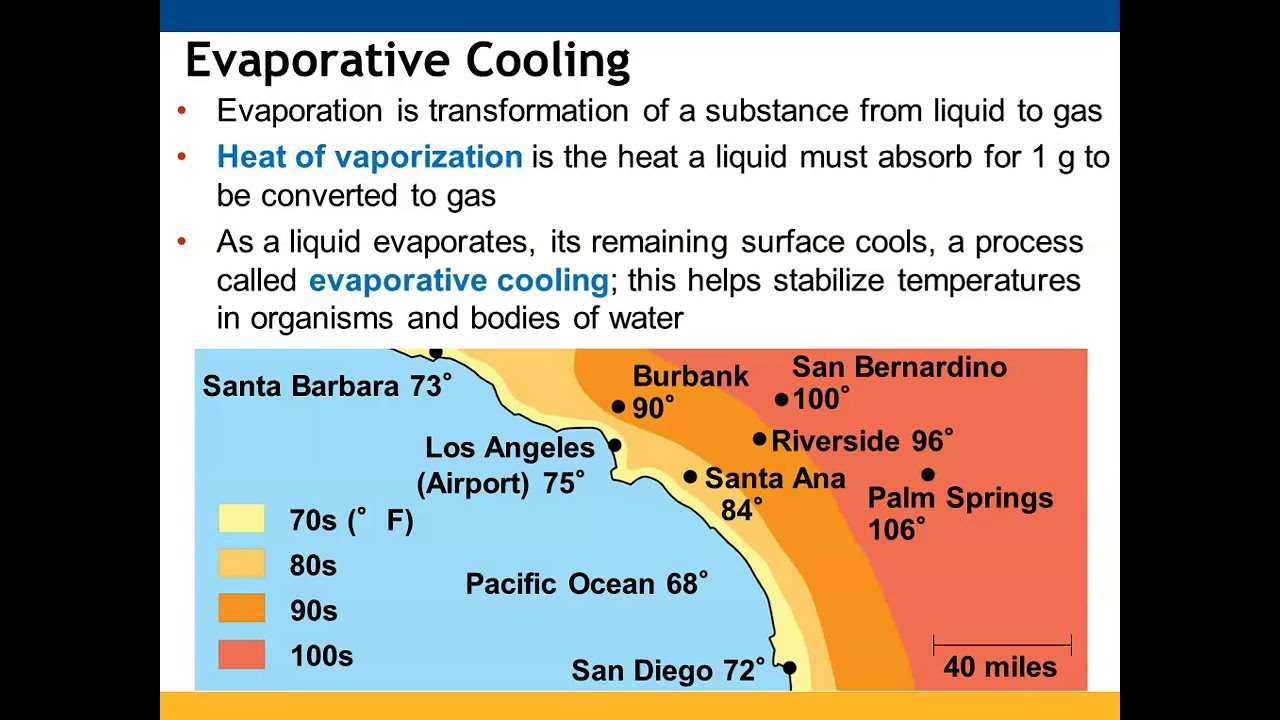
Enzymes are essential in ensuring that reactions occur at a pace suitable for life. By lowering the activation energy required for a reaction, enzymes increase the rate at which reactions happen without being consumed in the process. This efficiency allows for the rapid turnover of molecules, which is essential for maintaining the dynamic balance of a living organism.
Energy Flow in Biological Reactions
| Type of Reaction | Energy Involvement | Example |
|---|---|---|
| Exergonic | Energy released | Cellular respiration |
| Endergonic | Energy required | Photosynthesis |
| Redox | Electron transfer | Electron transport chain |
In summary, chemical reactions drive all processes necessary for survival, from energy production to the synthesis of complex molecules. Understanding the significance of these reactions in biology helps to explain how organisms grow, repair, adapt, and interact with their environments. These processes are fundamental to all living beings, linking the smallest molecules to the largest systems in an organism.