General Chemistry 2 Final Exam Questions and Answers

Success in your upcoming assessment depends on a solid understanding of key principles, along with the ability to apply them under pressure. The material covered in this course is both broad and intricate, requiring a focused approach to revision. By mastering core topics, you can approach each challenge with confidence.
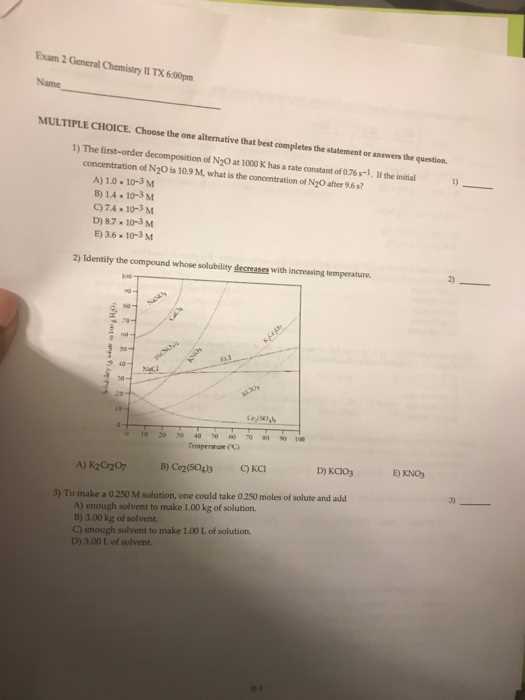
Understanding essential concepts will allow you to tackle various problems efficiently. From basic theories to more complex scenarios, practicing critical thinking and problem-solving techniques will prepare you for a range of tasks. It’s not only about memorizing formulas but also about knowing how to use them in real-world contexts.
Effective preparation involves reviewing practice sets, identifying common problem types, and focusing on areas that have historically been more challenging. By breaking down the material into manageable sections, you’ll be able to address each topic with clarity and precision.
General Chemistry 2 Final Exam Questions and Answers
This section will guide you through some of the most commonly encountered challenges in your upcoming assessment. It’s crucial to familiarize yourself with the concepts and problem-solving techniques that frequently appear in testing scenarios. Focusing on these will allow you to approach each task with greater efficiency and accuracy.
We’ve compiled a variety of sample problems that represent the key topics covered throughout the course. These exercises will help you solidify your understanding of complex theories and ensure you’re prepared to apply them effectively. Mastering these problem types will give you a strong foundation for tackling even the trickiest questions.
By working through these examples, you’ll develop the necessary skills to handle various problem-solving approaches, from balancing equations to interpreting data. This targeted practice is designed to help you feel confident in every aspect of the material when the time comes to face the test.
Key Concepts to Master for Chemistry Exams

To perform well on your upcoming assessment, it’s essential to grasp the core principles that form the foundation of the material. These central ideas will not only help you solve problems more efficiently but also allow you to approach complex topics with clarity. Understanding the broader concepts, rather than memorizing individual facts, will give you a more well-rounded preparation.
Understanding Atomic Structure

A solid understanding of atomic theory is crucial. This includes knowing how atoms interact, form bonds, and behave in different environments. Pay particular attention to the structure of the atom, electron configurations, and the periodic table’s organization. Mastery of this topic is vital for tackling questions related to bonding and molecular properties.
Mastering Thermodynamics and Kinetics
Energy transformations, entropy, and reaction rates are all key aspects to review. A deep understanding of thermodynamics will help you predict the direction of chemical reactions, while knowledge of kinetics will enable you to assess reaction speeds. Both topics are integral to comprehending how processes occur and the factors that influence them.
Focusing on these principles will ensure that you’re well-equipped to handle a variety of problems, from simple calculations to more complex theoretical scenarios. Through practice and consistent study, these concepts will become second nature, allowing you to confidently approach your test.
Top Questions Asked in Chemistry Finals
When preparing for your upcoming assessment, it’s helpful to focus on the types of challenges that frequently appear in testing scenarios. These common problem types are designed to assess your understanding of key concepts and your ability to apply them. By recognizing these patterns, you can streamline your study efforts and ensure you’re ready for a variety of topics.
Some of the most frequent problems involve balancing chemical reactions, calculating reaction rates, and understanding the behavior of gases. Additionally, questions related to stoichiometry and thermodynamic principles are staples of this subject area. Knowing how to approach each of these problem types will give you an edge when faced with similar scenarios.
Practice with diverse examples to develop a well-rounded grasp of the material. This approach will enable you to identify what the problem is asking, apply the correct formulas, and arrive at the solution quickly. Being prepared for these common topics will increase your confidence when tackling the test.
Essential Topics for Final Exam Preparation
To succeed in your upcoming test, it’s important to focus on the key subjects that are most likely to appear. These fundamental areas are critical for both understanding the material and being able to apply it effectively in problem-solving situations. Mastering these topics will ensure you are prepared for a wide range of tasks and challenges.
Key Areas in Organic Reactions
A strong grasp of organic reactions, including substitution and elimination processes, is essential. Understand the mechanisms behind each reaction and the factors that influence reaction rates. Being able to recognize reaction types and predict products is crucial for performing well in this area.
Understanding Gas Laws
Gas behavior and the application of various gas laws (Boyle’s Law, Charles’ Law, etc.) are fundamental topics. Knowing how to manipulate equations involving pressure, volume, and temperature will help you solve related problems efficiently. It’s also important to be familiar with real-world applications of these principles.
By focusing on these core areas, you’ll be better equipped to tackle the broad spectrum of topics that may arise. Effective preparation involves reviewing key concepts, practicing problems, and honing your ability to quickly recognize the best approach to any given question.
Study Strategies for General Chemistry 2
Effective preparation requires more than just reading through textbooks. To truly master the material, you need to adopt a structured approach that emphasizes both understanding and application. By focusing on key strategies, you can enhance your retention and problem-solving abilities, making it easier to tackle even the most challenging topics.
Active Learning Techniques
Rather than passively reviewing notes, engage with the material through active learning. This could include teaching concepts to a peer, solving practice problems, or using flashcards to test your recall. The more actively you engage with the subject, the better you’ll retain critical information and strengthen your understanding.
Consistent Practice and Review
Repetition is key to mastery. Set aside time each day to review different topics, ensuring you cover everything over multiple sessions. By regularly revisiting complex concepts, you’ll reinforce your understanding and increase your ability to apply them under pressure. Don’t skip the basics–even simple problems are essential for building a solid foundation.
Incorporating these techniques into your study routine will help you approach the material with confidence and clarity. Consistency and engagement are crucial to success, so make sure to stay focused and persistent in your preparation.
Understanding Chemical Reactions for Exams
A strong understanding of how substances interact and transform is crucial when preparing for assessments in this subject. Recognizing the different types of transformations, knowing how to predict products, and being able to balance equations are all key skills that will help you approach problems with confidence. These reactions often form the backbone of many challenges, so mastering them is essential for success.
Types of Chemical Reactions

There are several fundamental types of reactions you should be familiar with. Each one has distinct characteristics and patterns that can help you predict outcomes more effectively. Below is a table outlining the main types of reactions you’ll encounter:
| Type of Reaction | Description | Example |
|---|---|---|
| Synthesis | Two or more substances combine to form a single product. | A + B → AB |
| Decomposition | A single compound breaks down into two or more products. | AB → A + B |
| Single Replacement | One element replaces another in a compound. | A + BC → AC + B |
| Double Replacement | Two compounds exchange components to form two new compounds. | AB + CD → AD + CB |
| Combustion | A substance reacts with oxygen, often producing energy in the form of heat and light. | CH₄ + 2O₂ → CO₂ + 2H₂O |
Balancing Equations
Another essential aspect of chemical reactions is the ability to balance equations. This involves ensuring that the number of atoms on both sides of the reaction is equal, which is necessary to comply with the law of conservation of mass. Practice balancing simple and complex equations to increase your accuracy and speed when solving problems.
Understanding these types of reactions and how to work with them is key for success. By mastering reaction patterns and balancing techniques, you’ll be able to approach problems more systematically and effectively.
Common Mistakes in Chemistry Exams
When facing assessments in this field, it’s easy to make errors that stem from misunderstanding core concepts or rushing through problems. These mistakes often happen due to lack of attention to detail, incomplete knowledge, or simply being unfamiliar with the problem-solving process. Identifying these common errors in advance can help you avoid them and improve your performance.
Overlooking Units and Conversions
A frequent mistake is neglecting to account for units or improperly converting them during calculations. Many problems require you to convert between different units of measurement, such as grams to moles or liters to milliliters. Always double-check your units to ensure accuracy in your final result. Even a small error in unit conversion can lead to significant discrepancies in the answer.
Misinterpreting Reaction Types
Another common pitfall is misidentifying the type of reaction involved in a problem. Failing to recognize whether a reaction is synthesis, decomposition, or another type can lead to incorrect predictions or misapplication of formulas. Practice identifying reaction types and their specific characteristics to avoid confusion during your test.
By being mindful of these mistakes, you can approach the test with greater precision and confidence. Paying attention to detail, understanding the material fully, and practicing problem-solving techniques are key to minimizing errors and succeeding in your assessment.
How to Approach Chemistry Multiple-Choice Questions
Multiple-choice challenges are common in assessments, and mastering the technique to approach them effectively can significantly improve your performance. These types of questions require not only knowledge of the material but also the ability to analyze each option critically and efficiently. Developing a strategy for tackling these questions can save time and increase accuracy.
Read the Question Carefully
The first step in answering any multiple-choice problem is to carefully read the prompt. Often, questions contain key words or phrases that direct you toward the correct answer. Make sure to understand what the question is asking before looking at the possible answers, as this will prevent you from jumping to conclusions too quickly.
Eliminate Clearly Incorrect Options
A helpful strategy is to first eliminate answers that are obviously incorrect. This narrows down the options, increasing your chances of selecting the right one. Even if you’re unsure of the correct answer, removing unlikely choices gives you a better probability of guessing correctly. Be cautious, though–don’t eliminate answers based on assumptions without fully considering the question.
By following a structured approach and applying critical thinking to each option, you can enhance your chances of success with multiple-choice items. Always review your answers before finalizing them to ensure you haven’t missed any subtle details.
Practice Problems for General Chemistry 2
Practicing problems is a vital part of mastering the material. By working through various challenges, you can enhance your problem-solving skills and strengthen your understanding of key concepts. The more you practice, the more comfortable you will become with applying formulas, balancing reactions, and predicting outcomes.
Types of Practice Problems

Here are some common problem types you can practice to build your proficiency:
- Stoichiometry Problems: These involve converting between different units and substances in a chemical reaction.
- Reaction Mechanisms: Practice predicting the sequence of steps in a reaction and how intermediates form.
- Balancing Equations: Work on both simple and complex reactions to ensure you understand the law of conservation of mass.
- Molarity and Concentration Calculations: Solve problems related to solutions, including dilution and molarity.
Strategies for Solving Problems
When tackling these types of challenges, consider the following tips:
- Break Down the Problem: Read the problem carefully and identify what is being asked. Break the problem into smaller, manageable steps.
- Write Out Your Work: Always show your calculations. Writing down each step will help you catch errors and stay organized.
- Check Units and Significance: Ensure that units are consistent and that your answer makes sense in terms of magnitude and significance.
By regularly working through these practice problems, you’ll improve your speed, accuracy, and confidence when approaching similar challenges in assessments.
Reviewing Acid-Base Chemistry Concepts

Understanding the behavior of acids and bases is crucial for solving a wide range of problems. These concepts are fundamental in many areas of science, particularly when it comes to reactions and equilibrium. By reviewing key principles and reactions, you can develop a deeper understanding of how acids and bases interact and how to manipulate them in various contexts.
Key Concepts to Review
Focus on these core ideas to solidify your grasp of acid-base chemistry:
- Bronsted-Lowry Acid-Base Theory: Understanding the transfer of protons between substances is essential for identifying acids and bases.
- pH and pOH: Know how to calculate the acidity or basicity of a solution, and the relationship between pH, pOH, and concentration of ions.
- Strong vs. Weak Acids/Bases: Differentiate between substances that completely dissociate in water and those that only partially dissociate.
- Buffer Solutions: Learn how buffers work to maintain pH stability in solutions and the importance of their components.
Common Reactions Involving Acids and Bases
Several key reactions involve acids and bases that you should be familiar with:
- Neutralization Reactions: The reaction between an acid and a base to form water and a salt.
- Acid-Base Titrations: The process of determining the concentration of an unknown acid or base by neutralizing it with a standard solution.
- Amphoteric Substances: Understand how certain compounds, like water and amino acids, can act as both acids and bases depending on the environment.
By reviewing these topics, you’ll be better equipped to solve problems involving acid-base reactions and related calculations. A solid understanding of these principles is key to mastering this area of study.
Organic Chemistry in Final Exams

In assessments, understanding organic compounds and their reactions is critical for success. Organic reactions, molecular structures, and functional groups play a major role in many problems. By mastering these concepts, you can approach challenges with confidence and apply your knowledge effectively to various types of questions.
Key Organic Chemistry Topics to Master
Focus on the following areas to prepare for questions related to organic substances:
| Topic | Description |
|---|---|
| Functional Groups | Familiarize yourself with common groups like alcohols, acids, aldehydes, and amines. Recognizing these groups is key for identifying reactions. |
| Reaction Mechanisms | Understand common mechanisms such as nucleophilic substitution, elimination, and electrophilic addition. |
| Stereochemistry | Study the spatial arrangement of atoms and how it affects chemical properties and reactions, including chirality and isomerism. |
| Carbon Bonding | Understand the structure and bonding in organic molecules, including sigma and pi bonds, hybridization, and resonance. |
Tips for Tackling Organic Problems
When solving organic-related problems, keep these strategies in mind:
- Practice Reaction Pathways: Ensure you can predict the products of reactions by recognizing the type of mechanism involved.
- Draw Structures: Visual representation helps in understanding how atoms are bonded and how reactions proceed.
- Memorize Key Reactions: Some reactions are fundamental to understanding organic chemistry. Make sure to memorize the most common ones, like oxidation, reduction, and substitution reactions.
With a solid understanding of these areas, you’ll be well-equipped to tackle organic-related challenges effectively. Regular practice with reaction mechanisms and molecular structures will improve your speed and accuracy in assessments.
How to Solve Stoichiometry Questions
Solve quantitative problems by understanding the relationships between reactants and products in a chemical reaction. Stoichiometry involves calculating the amounts of substances involved using conversion factors. By mastering this process, you can determine how much of a substance is required or produced in a reaction.
Key Steps to Solve Stoichiometry Problems

Follow these essential steps when tackling stoichiometric calculations:
- Write a Balanced Equation: Ensure the reaction is properly balanced to correctly interpret the mole ratios between reactants and products.
- Convert to Moles: Convert the given information into moles using molar mass or other conversion factors.
- Use Mole Ratios: Use the coefficients from the balanced equation to convert between substances and relate the moles of reactants to products.
- Convert Back to Desired Units: If the problem asks for mass, volume, or other units, convert the result from moles back to the appropriate units.
Tips for Successful Calculations

Keep these strategies in mind to improve your problem-solving skills:
- Double-check Units: Always verify that your units cancel out correctly, leading to the desired unit for the final answer.
- Stay Organized: Break down complex problems into smaller, manageable steps to avoid confusion.
- Practice Regularly: The more you practice stoichiometry problems, the quicker and more accurate you’ll become.
By following these steps and refining your calculation techniques, you can approach stoichiometric problems with confidence and accuracy in any test or assessment.
Tips for Balancing Chemical Equations
Successfully balancing a reaction involves ensuring that the number of atoms of each element is the same on both sides of the equation. It is a fundamental skill that allows accurate representation of chemical transformations. Here are some essential strategies to master this process effectively.
Step-by-Step Approach to Balancing
Follow these steps to balance equations efficiently:
- Start with the Most Complex Compound: Begin by balancing the elements in the most complex molecule, typically the one with the most atoms.
- Balance Atoms One Element at a Time: After addressing the complex compound, balance each element individually. Keep track of the number of atoms on both sides of the equation.
- Balance Hydrogen and Oxygen Last: These elements are often present in multiple compounds, so it’s best to leave them until the other elements are balanced.
- Use Coefficients, Not Subscripts: Adjust the coefficients in front of compounds to balance atoms, rather than changing the subscripts within the molecules.
Helpful Tips for Success
- Check Your Work: After balancing, recount the atoms of each element on both sides to ensure they match.
- Be Patient: Balancing equations can be challenging at first, but with practice, the process becomes more intuitive.
- Use Fractions if Needed: Sometimes using fractions for coefficients can make balancing easier, and you can multiply the entire equation by a common denominator at the end to eliminate them.
Balancing reactions takes practice, but applying these strategies will help simplify the process and build your confidence in handling different types of chemical equations.
Analyzing Periodic Trends for Finals
Understanding the periodic behavior of elements is crucial for mastering many aspects of the subject. Periodic trends are patterns observed in the properties of elements as you move across periods or down groups in the periodic table. Recognizing these patterns will not only deepen your understanding but also help you tackle related problems in assessments.
Key Periodic Trends to Focus On
There are several important trends to examine as you prepare:
- Atomic Radius: As you move across a period from left to right, the atomic radius decreases due to increasing nuclear charge. Conversely, as you move down a group, the atomic radius increases as additional electron shells are added.
- Ionization Energy: This is the energy required to remove an electron from an atom. Ionization energy tends to increase across a period and decrease down a group.
- Electronegativity: This refers to the tendency of an atom to attract electrons in a bond. Electronegativity increases across a period and decreases down a group, with fluorine being the most electronegative element.
- Electron Affinity: Electron affinity refers to the energy change when an atom gains an electron. This value typically becomes more negative across a period, reflecting a greater tendency to accept an electron.
How to Use These Trends for Problem Solving
Knowing the direction and behavior of these trends can guide you in predicting the properties of elements and ions. Here are a few strategies:
- Predicting Reactivity: Elements with low ionization energies, such as alkali metals, are highly reactive, while those with high electronegativities, like halogens, tend to form strong bonds.
- Comparing Elements: Use periodic trends to compare elements within a period or group. For example, when comparing metals and non-metals, the difference in electronegativity and ionization energy becomes significant.
- Answering Conceptual Questions: Many questions will test your ability to apply these trends in theoretical scenarios. Practice identifying how changes in atomic structure affect chemical behavior.
By focusing on these fundamental trends and applying them to real problems, you will gain a deeper understanding of elemental behavior and be better prepared for assessments. Keep practicing, and over time, recognizing these trends will become second nature.
Effective Time Management During Exams
Managing time effectively during an assessment is crucial for success. It is important to approach each task systematically, ensuring that all sections are addressed while maintaining a steady pace. Strategic planning helps reduce stress and ensures that all questions are answered thoughtfully without rushing or neglecting key details.
The first step in effective time management is to quickly assess the overall structure of the assessment. Familiarize yourself with the number of questions, their formats, and their allocated point values. This allows you to prioritize tasks according to their complexity and importance. Divide your time in a way that gives more attention to questions with higher point values or those requiring more in-depth reasoning.
Another key element is setting time limits for each section. For example, allocate a fixed amount of time to each question or problem, ensuring that you are not spending too much time on any one item. If you encounter a particularly difficult question, it may be wise to move on and return to it later if time permits.
When possible, practice under timed conditions prior to the assessment. This helps develop a sense of how long you should spend on each type of question and trains your brain to work efficiently. During practice sessions, simulate real exam conditions, avoid distractions, and track your progress to gauge your time management skills.
Finally, keep an eye on the clock during the test. It is essential to stay aware of time without obsessing over it. Make sure you pace yourself throughout the entire duration, leaving enough time at the end to review your answers and make any necessary corrections.
Reviewing Thermodynamics and Kinetics
Understanding the principles governing energy changes and reaction rates is fundamental to mastering many concepts. The study of how energy flows within a system and how molecules interact over time plays a key role in predicting the behavior of reactions. These areas are crucial not only for academic assessments but also for real-world applications in various scientific fields.
Thermodynamics deals with the direction of energy transfer and the conditions under which reactions are favorable or spontaneous. It focuses on concepts such as enthalpy, entropy, and Gibbs free energy, which determine whether a process will occur naturally. Familiarity with these principles helps in predicting the spontaneity of reactions and understanding equilibrium.
On the other hand, kinetics explores the rates at which reactions proceed. Key factors such as activation energy, reaction mechanisms, and the influence of temperature and concentration are essential for understanding how quickly reactions occur. Kinetic studies help explain why some reactions proceed rapidly while others take much longer to reach completion.
Reviewing these topics involves grasping both the theoretical foundations and the ability to apply them to problem-solving. Practice solving problems related to energy changes and reaction rates, focusing on concepts like the Arrhenius equation and Le Chatelier’s principle. Mastering these areas will provide a solid foundation for tackling more complex scenarios in the subject.