Flame Tests Lab Answer Key for Chemistry Experiments
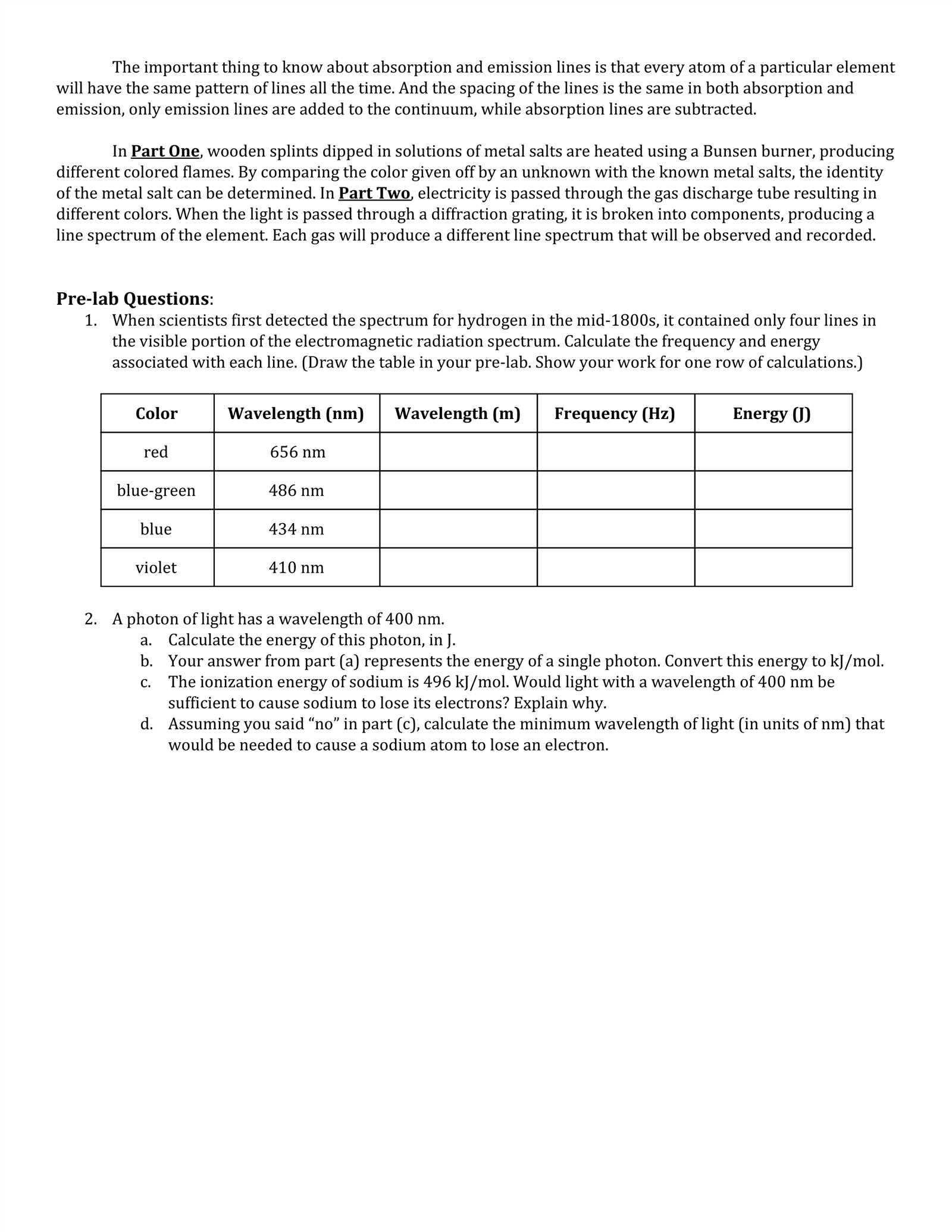
In chemistry, identifying different elements often involves observing their reactions when exposed to heat. This method is essential for distinguishing various substances based on the colors they emit under specific conditions. By performing these procedures, students and scientists can gain valuable insights into the chemical composition of unknown samples.
In this section, we will explore how various elements react to heat and the specific colors that are produced during these reactions. Knowing these reactions is crucial for qualitative analysis, allowing individuals to identify materials with precision and accuracy. The process of interpreting these color changes plays a vital role in modern chemical experiments and analysis.
Whether you are a beginner or an experienced practitioner, understanding these fundamental techniques is a key aspect of mastering basic chemical methods. Through careful observation and accurate interpretation of the results, you can enhance your skills in chemical analysis.
Flame Tests Lab Answer Key Overview
In chemical analysis, one of the key methods for identifying substances involves observing the distinct colors produced when certain materials are heated. This approach allows for the identification of metal ions based on their unique color emissions under controlled conditions. The concept behind these reactions is fundamental in various scientific applications, providing essential information about the material being studied.
Purpose of Heat-Induced Color Reactions
These color changes are not random; they correspond to the specific energy levels of electrons within the atoms of the element being heated. When these electrons absorb energy, they move to higher energy states, and as they return to their original levels, they release light at specific wavelengths. This light is visible as color, and each metal ion emits a characteristic hue, making it possible to distinguish between different substances based on the observed color.
Applications in Qualitative Analysis
This method is particularly useful in qualitative analysis, where the goal is to identify the chemical composition of a substance rather than quantify its amount. By using this technique, chemists can quickly determine the presence of certain elements in unknown samples, which is crucial in various fields such as forensic science, material analysis, and environmental studies.
Flame Tests Lab Answer Key Overview
In chemical analysis, one of the key methods for identifying substances involves observing the distinct colors produced when certain materials are heated. This approach allows for the identification of metal ions based on their unique color emissions under controlled conditions. The concept behind these reactions is fundamental in various scientific applications, providing essential information about the material being studied.
Purpose of Heat-Induced Color Reactions
These color changes are not random; they correspond to the specific energy levels of electrons within the atoms of the element being heated. When these electrons absorb energy, they move to higher energy states, and as they return to their original levels, they release light at specific wavelengths. This light is visible as color, and each metal ion emits a characteristic hue, making it possible to distinguish between different substances based on the observed color.
Applications in Qualitative Analysis
This method is particularly useful in qualitative analysis, where the goal is to identify the chemical composition of a substance rather than quantify its amount. By using this technique, chemists can quickly determine the presence of certain elements in unknown samples, which is crucial in various fields such as forensic science, material analysis, and environmental studies.
How to Perform a Flame Test
Conducting a procedure to observe the color changes when substances are exposed to intense heat is an essential technique in chemistry. This process helps identify certain elements based on their characteristic glow when they react with high temperatures. The process is straightforward and can be done with basic equipment to uncover important information about the sample being studied.
Required Materials
| Item | Purpose |
|---|---|
| Bunsen burner | Source of heat |
| Metal loop or wire | To hold the substance |
| Chemical samples | Substances to be analyzed |
| Distilled water | For cleaning the loop |
| Heat-resistant gloves | For safety precautions |
Step-by-Step Procedure
Begin by securing the sample on the metal loop or wire. Ensure that the equipment is clean to avoid contamination. Next, adjust the burner to a steady blue flame. Carefully bring the sample into the center of the flame. Observe the color change produced by the reaction. Different elements will produce distinct hues, which can be recorded for further analysis.
Importance of Flame Colors in Chemistry
The observation of color changes when certain materials are subjected to heat is a valuable tool in the field of chemistry. These color shifts are directly linked to the presence of specific elements and can be used to identify their characteristics. By understanding the relationship between color and chemical composition, scientists can gain insights into the nature of substances and their reactions under high temperatures.
Identification of Elements
Each element emits a unique spectrum of light when heated, resulting in specific colors. These colors act as a visual fingerprint, allowing chemists to distinguish between different substances. The patterns of light observed provide crucial information about the atomic structure and the energetic behavior of the element in question.
Applications in Analytical Chemistry
This technique is widely used in various analytical methods, such as qualitative analysis, where it aids in detecting the presence of metals and other compounds in samples. The distinct colors produced under heat can help verify the composition of a substance, making this method essential for both educational purposes and practical laboratory analysis.
Identifying Metal Ions with Flame Tests
The process of observing the colors produced when various substances are exposed to intense heat is an effective method for identifying specific metal ions. These ions, when heated, emit distinct colors, allowing chemists to determine the presence of particular elements in a sample. The color produced by a sample is closely linked to the energy transitions that occur within the metal ions’ electrons under high temperature conditions.
Common Metal Ions and Their Color Reactions
- Lithium (Li+): Crimson red
- Sodium (Na+): Bright yellow
- Potassium (K+): Lilac or light purple
- Calcium (Ca2+): Orange-red
- Barium (Ba2+): Green
- Copper (Cu2+): Blue-green
Steps for Identification
- Prepare a clean metal wire or loop to hold the sample.
- Dip the wire into a small amount of the substance to be tested.
- Heat the sample in the flame and observe the resulting color.
- Compare the observed color to known standards for various metal ions.
Safety Guidelines for Flame Tests
When performing procedures involving intense heat and chemicals, it is crucial to follow proper safety measures to ensure a safe environment for both the experimenter and others nearby. Exposure to high temperatures, harmful substances, and open flames can present various risks. By adhering to the appropriate precautions, potential hazards can be minimized, ensuring a secure working environment.
| Safety Measure | Description |
|---|---|
| Wear Protective Gear | Always wear safety goggles, heat-resistant gloves, and a lab coat to protect against burns, splashes, and flying debris. |
| Use a Fume Hood | Perform the procedure in a well-ventilated area, preferably a fume hood, to avoid inhaling harmful fumes or gases produced during heating. |
| Control Open Flames | Keep flames at a safe distance from flammable materials. Always extinguish the flame when it is not in use. |
| Handle Chemicals Carefully | Use caution when working with chemical samples, and ensure they are stored and disposed of properly according to safety guidelines. |
| Know Emergency Procedures | Familiarize yourself with the location and use of fire extinguishers, eyewash stations, and emergency exits in case of an incident. |
Interpreting Flame Test Results Accurately
Properly analyzing the colors produced when certain substances are heated is essential for identifying the metal ions present. The colors observed during heating can provide valuable insights into the chemical composition of a sample. However, accurate interpretation requires attention to detail, as several factors can influence the results, such as the purity of the sample and the intensity of the heat applied.
Key Factors to Consider
- Temperature Variations: Different temperatures can affect the intensity and shade of the color emitted, so consistent heating is crucial for reliable results.
- Sample Purity: Contaminants in the sample may alter the color produced, making it important to ensure that the substance being tested is pure.
- Lighting Conditions: Ambient lighting can sometimes make it harder to differentiate between subtle color changes. It’s best to perform the procedure in a controlled environment with minimal external light.
Comparing Results
Once the color is observed, it should be compared to known standards for different elements. Each element produces a characteristic color when heated, and recognizing these hues allows for the identification of the metal ions present. However, cross-referencing with reliable sources or reference charts is key to confirming the results.
Common Errors in Flame Test Procedures
When performing procedures that involve heating substances to observe color changes, several mistakes can hinder the accuracy of the results. These errors may arise from improper technique, equipment issues, or environmental factors. Understanding these common pitfalls can help ensure that the process is conducted correctly and the results are reliable.
Improper Sample Handling
One of the most frequent mistakes is inadequate cleaning of the metal loop or wire used to hold the sample. Residue from previous experiments can contaminate the sample and alter the expected color. Always ensure the wire is thoroughly cleaned with distilled water before use. Failing to do so can lead to misleading results.
Incorrect Heat Application
- Uneven Heating: If the sample is not placed in the center of the heat source, it may not reach the correct temperature, leading to faint or incorrect color emissions.
- Insufficient Heat: Using a flame that is too weak may not excite the electrons in the metal ions enough to produce the characteristic color.
- Excessive Heating: Overheating can cause decomposition of the sample, which can affect the color produced or even cause the substance to burn away.
Flame Test Answer Key for Common Elements
In experiments involving the heating of substances, the colors produced can provide valuable insights into the presence of specific metal ions. Each element, when exposed to heat, emits a unique color that can help identify it. The following guide outlines the typical color emissions associated with some of the most common elements found in such procedures.
- Lithium (Li+): Crimson red
- Sodium (Na+): Bright yellow
- Potassium (K+): Light purple or lilac
- Calcium (Ca2+): Orange-red
- Barium (Ba2+): Green
- Copper (Cu2+): Blue-green
- Strontium (Sr2+): Red
- Iron (Fe3+): Yellow-brown
Factors Affecting Flame Test Accuracy
Several factors can influence the precision and reliability of results when heating substances to observe the colors they emit. Variations in technique, equipment, and environmental conditions all play a role in determining whether the observed color accurately represents the substance being analyzed. Being aware of these factors is crucial for obtaining valid results.
Key Factors
- Sample Contamination: Impurities in the sample can lead to unexpected color results. Ensuring that the sample is pure and free from contamination is vital for accurate identification.
- Heat Intensity: The temperature of the heat source is critical. Insufficient heating may not excite the electrons enough to produce a clear color, while excessive heat can decompose the sample and obscure the results.
- Sample Size: The amount of substance used can affect the intensity of the color. A very small sample may produce a faint or unclear emission, while too large a sample could result in a mixture of colors.
- Lighting Conditions: External light, such as overhead or ambient lighting, can interfere with color observation. Conducting the procedure in a controlled, dimly lit environment helps to see the color clearly.
- Equipment Cleanliness: Residue from previous substances on the heating wire or loop can alter the color results. It’s important to clean all equipment thoroughly between each use.
Ensuring Accurate Results
- Use a clean wire or loop to avoid cross-contamination between samples.
- Maintain a consistent heat source to ensure reliable and repeatable results.
- Perform the procedure in a controlled environment with minimal light interference.
- Use a sufficient amount of the sample for a strong and visible color emission.
Flame Test Answer Key for Sodium and Potassium
The colors produced when heating sodium and potassium salts can serve as a quick and reliable method for identifying these elements in a sample. Each of these metals emits a distinctive hue when exposed to heat, making it easy to differentiate between them. Understanding the characteristic colors of sodium and potassium under high temperature conditions is essential for accurate identification.
- Sodium (Na+): Bright yellow
- Potassium (K+): Light purple or lilac
Using Flame Tests for Qualitative Analysis
In analytical chemistry, identifying the presence of specific elements in a sample can often be done by observing the colors emitted when the sample is heated. This method is especially useful for detecting metal ions, as each element produces a unique color under heat. These observations can provide valuable qualitative data, allowing chemists to confirm the composition of an unknown substance.
Procedure Overview
To conduct an analysis, a small sample of the substance is introduced to a heat source. The resulting color is then compared to known standards to identify the metal ions present. This method works because different elements have electrons that are excited to higher energy levels when heated, and as they return to their original state, they release energy in the form of visible light with a specific wavelength, corresponding to distinct colors.
Advantages of This Approach
- Speed: The process is quick and can provide immediate results, making it ideal for rapid analysis in a variety of situations.
- Simplicity: The method is relatively simple to perform, requiring minimal equipment and expertise.
- Cost-Effective: It does not require expensive reagents or complex instruments, making it a cost-effective option for qualitative analysis.
Flame Test Answer Key for Calcium and Copper
The colors produced when heating calcium and copper salts can provide important clues for identifying these elements in a sample. By observing the specific hues emitted during the heating process, it is possible to distinguish calcium from copper and gain insight into the chemical composition of the substance being tested.
- Calcium (Ca2+): Orange-red
- Copper (Cu2+): Blue-green
Flame Test Variations and Modifications
The process of observing colors emitted by heated substances can be adjusted or refined to suit different experimental conditions or to improve accuracy. Variations in technique or modifications to the procedure can help optimize the results or extend the range of elements that can be identified. By understanding and implementing these changes, the effectiveness of the process can be enhanced in various analytical situations.
Modifications for Enhanced Accuracy
- Using Different Heat Sources: Substituting a stronger or more consistent heat source can improve the visibility of colors, especially for elements that emit faint hues.
- Adjusting Sample Size: A larger or more concentrated sample may result in a more vivid color emission, improving identification accuracy.
- Adding Chemicals to Intensify Colors: Some procedures involve introducing specific chemicals to increase the intensity of the color produced by certain metal ions.
Variations for Specific Elements
- Using Specific Wire Materials: Switching from a standard metal wire to one made from a more chemically resistant material, like platinum, can prevent contamination and ensure cleaner results.
- Changing the Environment: Performing the procedure in a controlled atmosphere, such as in a vacuum or with controlled humidity, may reduce the interference of environmental factors and enhance precision.
Advanced Applications of Flame Tests
The observation of colors emitted by heated substances is not only a fundamental technique in chemistry but also has advanced applications in various fields. This method is valuable for both qualitative and quantitative analysis, extending its utility to industries such as forensic science, material analysis, and environmental monitoring. Understanding the nuances of this technique allows for more sophisticated use in different scientific and industrial contexts.
Applications in Industry and Research
- Environmental Monitoring: Detecting trace amounts of metal ions in air or water samples is crucial for environmental safety. This method can be used to quickly identify pollutants like lead, copper, or zinc in the environment.
- Forensic Science: The technique can help identify materials found at crime scenes, such as residues from metal-based compounds in explosives or weapons.
- Material Analysis: In the field of materials science, it is used to analyze the composition of metals and alloys, providing valuable information for quality control and manufacturing processes.
Enhancing Sensitivity and Specificity
- Combination with Spectroscopy: By pairing this technique with advanced spectroscopic methods, researchers can achieve even greater precision in identifying elements and their concentrations in complex samples.
- High-Throughput Screening: In industrial applications, the method can be automated for high-throughput screening of large quantities of samples, making it more efficient for quality assurance and rapid analysis.
- Trace Element Analysis: In highly sensitive applications, modifications such as the use of specific flame conditions or additional chemical reagents can enhance the detection limits for trace elements in complex mixtures.
Reviewing Flame Test Results in Labs
When conducting experiments that involve observing the colors emitted by heated substances, it is important to carefully analyze and interpret the results. The ability to identify distinct colors produced by various elements is key to understanding the composition of unknown samples. Accurate observations and comparisons to known standards are essential for ensuring reliable conclusions.
Critical Factors to Consider: Several factors can influence the outcome of these experiments. These include the concentration of the sample, the type of heat source used, and even environmental conditions such as humidity or atmospheric interference. Understanding these variables helps prevent misinterpretation of results.
Common Errors in Analysis: One common mistake is failing to account for the intensity of the color produced. Some elements emit very faint or subtle hues, which might be overlooked if not carefully observed. Additionally, cross-contamination between samples or incorrect handling of materials can lead to inaccurate readings, making it important to follow proper protocols.
Steps for Accurate Review:
- Ensure all equipment is clean and free from contaminants.
- Compare the observed color with a comprehensive reference chart under consistent lighting conditions.
- Record the results systematically, noting any variations in hue or intensity that might indicate the presence of specific elements.
- Repeat the experiment as needed to confirm initial findings, particularly for elements with weak or ambiguous colors.