2004 AP Chemistry Free Response Answers Form B
The AP exam is a crucial step for students aiming to demonstrate their knowledge in advanced scientific subjects. One of the most challenging aspects of the exam is tackling complex questions that require more than simple recall. These types of tasks demand clear problem-solving skills and the ability to apply concepts in unfamiliar scenarios.
In this section, we will explore detailed solutions and step-by-step explanations for some of the most challenging tasks from a past exam. By breaking down each question, we aim to provide insights into the logical process and techniques needed to arrive at the correct conclusions. Whether you are preparing for your first test or refining your exam strategy, understanding how to approach these kinds of problems is essential.
Key topics will be highlighted, and essential strategies for efficient problem-solving will be covered to help students boost their performance. This guide serves as a valuable tool for those looking to deepen their understanding of the subject and improve their approach to similar exam challenges.
2004 AP Chemistry Free Response Answers Form B
This section covers the breakdown of the challenging written tasks from the advanced scientific exam, providing insights into the problem-solving process. These problems are designed to assess the depth of knowledge and the ability to apply theoretical concepts in practical situations. By working through these exercises, students can gain a better understanding of how to structure their responses and approach similar questions in the future.
Key Strategies for Approaching Complex Problems
When faced with intricate questions, it’s essential to have a systematic approach. Here are some tips to keep in mind:
- Read each question thoroughly to understand its context.
- Identify the key principles or formulas that are relevant to the problem.
- Break down the question into smaller, manageable parts.
- Write your response clearly, showing all steps to reach the conclusion.
- Double-check your calculations and reasoning to ensure accuracy.
Breaking Down Specific Questions
Let’s examine a few examples of challenging tasks from the exam. Each of these questions tests different aspects of scientific understanding, and approaching them with a structured methodology will improve your chances of success.
- Task 1: Analyzing a chemical reaction and determining the rate of change.
- Task 2: Calculating energy changes during a reaction.
- Task 3: Interpreting experimental data and drawing conclusions based on trends.
By practicing such tasks, students can build the necessary skills to navigate similar problems effectively and improve their overall exam performance.
Overview of AP Chemistry Exam Format
The advanced science exam is structured to evaluate a student’s understanding of key concepts and their ability to apply them in real-world contexts. The test is divided into two main sections: multiple-choice questions and written tasks. Each section is designed to assess different aspects of knowledge and problem-solving skills, with an emphasis on both theoretical and practical understanding.
The multiple-choice portion is focused on testing a student’s grasp of fundamental principles, requiring quick decision-making and the ability to select the correct answer from a range of options. The written tasks, on the other hand, demand more comprehensive responses where students must explain their reasoning, solve complex problems, and demonstrate their ability to analyze data and interpret scientific scenarios.
Both sections of the exam are time-bound, meaning that efficient time management is crucial for success. Students must be able to allocate their time wisely between answering questions and carefully reviewing their work to ensure accuracy in their responses. Preparation for the exam requires not only mastering the material but also becoming familiar with the exam format and the types of questions that may appear.
Understanding the Free Response Section
The written portion of the advanced science exam is designed to evaluate how well students can apply their knowledge in complex, real-world scenarios. Unlike multiple-choice questions, these tasks require detailed explanations and a step-by-step approach to problem-solving. This section tests not only the depth of understanding but also the ability to communicate scientific reasoning clearly and effectively.
To excel in this part of the exam, students must be prepared to address a variety of challenges. The questions often require critical thinking, data analysis, and synthesis of various concepts. Here are some key points to keep in mind when approaching this section:
- Clarity in explanation: Every step of your solution should be well-organized and clearly written.
- Structured approach: Break down the problem into smaller parts and tackle each step systematically.
- Show all work: Be sure to display all calculations and thought processes to demonstrate your reasoning.
- Stay on topic: Keep responses focused on the question, avoiding irrelevant details.
- Check your work: Always review your answers to ensure that no steps are missed and that the reasoning is sound.
By practicing these strategies, students can better navigate the challenges presented in this section and improve their overall performance on the exam.
Tips for Answering Free Response Questions
The written section of the advanced exam requires a different approach compared to multiple-choice questions. These tasks demand clear, concise, and logical explanations, where students need to showcase their ability to apply scientific principles to complex problems. Success in this part of the exam depends on careful preparation and effective problem-solving strategies.
Here are some helpful tips to keep in mind when tackling written questions:
- Read the question carefully: Ensure you understand exactly what is being asked before starting your response.
- Highlight key terms: Identify important concepts or data in the question that will guide your solution.
- Organize your thoughts: Break the problem into smaller steps and address each part clearly and logically.
- Show your work: Always provide a detailed explanation of how you arrived at your solution, including any calculations and reasoning.
- Use proper units: Be consistent with units and ensure that all quantities are appropriately labeled.
- Stay focused: Avoid irrelevant information and stick to the question’s requirements.
- Review your response: Take a moment to check for mistakes, missing steps, or errors in your logic.
By practicing these strategies, you will develop the skills needed to approach written questions with confidence, ensuring that you can communicate your understanding clearly and accurately.
How to Approach Chemical Reaction Questions
Questions involving chemical reactions require a thorough understanding of reaction mechanisms, stoichiometry, and the principles governing the behavior of matter. These tasks often present scenarios where students must analyze reactions, predict outcomes, and perform calculations based on given data. To succeed, it’s essential to approach these questions with a clear strategy and a solid grasp of the underlying concepts.
Steps to Tackle Chemical Reaction Problems
When faced with a question about a chemical reaction, follow these key steps to break down the problem and reach an accurate solution:
- Write out the balanced equation: Ensure the chemical equation is properly balanced to reflect the conservation of mass.
- Identify the type of reaction: Determine whether the reaction is a synthesis, decomposition, combustion, etc., as this can guide your approach.
- Determine the limiting reactant: If applicable, identify which reactant will run out first and limit the amount of product formed.
- Apply stoichiometry: Use molar ratios from the balanced equation to calculate the amount of products or reactants involved.
- Consider reaction conditions: Take into account temperature, pressure, or other factors that may influence the outcome of the reaction.
Common Pitfalls to Avoid
While solving these problems, it’s important to watch out for common errors that could lead to incorrect answers:
- Neglecting to balance the equation: An unbalanced equation will lead to incorrect calculations and a misunderstanding of the reaction’s behavior.
- Ignoring units: Always include and convert units properly to avoid mistakes in stoichiometric calculations.
- Forgetting significant figures: Be mindful of significant figures in your final answer, especially when working with measured quantities.
By following these guidelines, students can effectively approach and solve chemical reaction-related questions with confidence and precision.
Strategies for Time Management During the Exam
Time management is a crucial skill for succeeding in any timed exam. The ability to allocate your time wisely ensures that you can answer all questions thoughtfully without rushing or leaving any unfinished. In exams with both multiple-choice and written tasks, balancing the time spent on each section is essential to maximizing your performance.
Effective Time Allocation
One of the most important strategies is learning how to distribute your time based on the difficulty and length of each section. Here are some techniques to help you manage your time effectively:
- Read through the entire exam: Quickly glance through the entire test to get a sense of the types of questions and their complexity.
- Set time limits for each section: Divide your available time between the multiple-choice and written parts. Be strict with yourself to stay within these limits.
- Start with easier questions: Tackle questions you find easier first, ensuring you secure points before moving to the more challenging ones.
- Leave difficult questions for later: If a question is taking too long, move on and come back to it after completing others.
- Allocate time for review: Set aside the last few minutes to review your answers, especially the written tasks, to catch any mistakes.
Staying Calm Under Pressure

Time pressure can lead to stress, which often affects your performance. Staying calm and focused is key to managing your time effectively. Consider these tips:
- Practice under timed conditions: Simulate exam conditions during your study sessions to build confidence in managing time.
- Take deep breaths: If you feel anxious, pause for a moment, take deep breaths, and refocus before continuing.
- Maintain a steady pace: Avoid rushing or overthinking. Keep a steady rhythm and trust your preparation.
By applying these time management strategies, you can ensure that you have enough time to thoroughly address each task, review your responses, and leave the exam feeling confident.
Common Mistakes to Avoid in Free Responses
When tackling written questions on an exam, it’s easy to make small errors that can have a significant impact on your score. These mistakes can arise from misunderstandings of the question, careless miscalculations, or failure to fully explain your reasoning. Avoiding common pitfalls is essential for maximizing your performance and ensuring that your responses are clear, complete, and accurate.
Here are some typical mistakes that students often make and tips on how to avoid them:
- Failing to explain your reasoning: One of the most common errors is not providing enough detail or context in your explanation. Always show how you arrived at your answer, even if the steps seem obvious to you.
- Skipping necessary steps: It’s tempting to jump straight to the final answer, but skipping steps can lead to missed points. Always include all intermediate steps, calculations, or justifications, even if they seem minor.
- Incorrect unit conversions: A simple error in unit conversion can throw off your entire calculation. Double-check your units and make sure they match the quantities you’re working with.
- Not reviewing your work: It’s easy to overlook mistakes in the heat of the moment. If time permits, take a moment to reread your responses and check for any errors, missing information, or unclear steps.
- Misunderstanding the question: Take the time to read the question carefully and make sure you understand exactly what is being asked. Misinterpreting the prompt can lead to irrelevant or incomplete answers.
- Being too brief: Some students provide overly concise answers that don’t fully address the question. While brevity is important, make sure your response is comprehensive and covers all aspects of the question.
By avoiding these mistakes and taking the time to carefully prepare and review your responses, you can significantly improve the quality of your work and increase your chances of scoring higher on written portions of the exam.
Key Concepts Tested in 2004 Form B
The exam evaluates a variety of fundamental scientific principles that test students’ ability to apply knowledge in practical scenarios. These topics cover a wide range of areas within the subject, focusing on both theoretical understanding and problem-solving skills. Below is an overview of the primary concepts that are frequently tested in this assessment, highlighting the knowledge necessary for success.
Each concept is crucial for tackling complex questions, and mastering these areas will significantly improve performance. The following table outlines the key concepts along with their associated skills:
| Concept Area | Description | Skills Tested |
|---|---|---|
| Chemical Reactions | Understanding the various types of reactions and predicting products based on given reactants. | Identifying reaction types, balancing equations, predicting products |
| Stoichiometry | Using mole ratios and conversion factors to determine quantities in chemical reactions. | Calculating molar relationships, determining limiting reagents, reaction yield |
| Thermodynamics | Investigating energy changes in reactions, including concepts of heat and work, enthalpy, and entropy. | Calculating energy changes, understanding the first and second laws of thermodynamics |
| Kinetics | Exploring factors influencing the rate of reactions such as concentration, temperature, and catalysts. | Determining reaction order, calculating rate constants, understanding activation energy |
| Equilibrium | Analyzing how reversible reactions reach equilibrium and how external changes can shift the position. | Applying Le Chatelier’s principle, calculating equilibrium constants, analyzing concentration changes |
| Electrochemistry | Studying the relationship between chemical reactions and electrical energy, including galvanic and electrolytic cells. | Calculating cell potentials, understanding oxidation-reduction reactions, applying the Nernst equation |
Mastering these key concepts will allow students to approach problems with confidence and accuracy, improving both their theoretical understanding and practical problem-solving abilities.
Detailed Breakdown of Each Question
This section provides a comprehensive analysis of each question from the assessment, breaking down the key elements and identifying the types of skills required to answer them effectively. By understanding the structure and focus of each query, students can better prepare for similar challenges in future evaluations.
Question 1: Reaction Mechanisms and Predictions
This question typically involves analyzing a given chemical reaction and predicting the products based on reactants and conditions. It tests your understanding of reaction types, balancing equations, and the ability to predict how changes in temperature or concentration affect the outcome. In order to answer this question effectively, students should:
- Identify the type of reaction (e.g., synthesis, decomposition, redox, etc.).
- Balance the equation correctly, ensuring the law of conservation of mass is adhered to.
- Apply knowledge of reaction mechanisms to predict the products under specified conditions.
Question 2: Thermodynamic Calculations
This section evaluates a student’s understanding of energy changes during chemical reactions, focusing on enthalpy, entropy, and Gibbs free energy. Students are often required to calculate the heat of reaction or the equilibrium constant based on provided data. To solve this question, students should:
- Understand the relationship between temperature, pressure, and the spontaneity of reactions.
- Use standard enthalpy and entropy values to calculate changes in Gibbs free energy.
- Interpret the significance of negative and positive values of ΔG in determining reaction feasibility.
Question 3: Kinetics and Rate Laws
Questions in this section generally require students to determine the rate law for a given reaction, analyze reaction order, and calculate the rate constant. You may also be asked to discuss factors that influence reaction rates. Key areas to focus on include:
- Determining the order of reaction with respect to each reactant.
- Using experimental data to calculate the rate constant (k).
- Understanding the effect of temperature, concentration, and catalysts on reaction rates.
Question 4: Electrochemical Cells
Here, the focus is on understanding the principles of electrochemistry, including the operation of galvanic and electrolytic cells. Students are usually required to calculate cell potentials and apply the Nernst equation. Important steps include:
- Balancing half-reactions for oxidation and reduction processes.
- Calculating the standard electrode potential and using it to determine the overall cell potential.
- Understanding how changing concentrations of reactants or products influences the cell potential using the Nernst equation.
Question 5: Equilibrium and Le Chatelier’s Principle
This question assesses the ability to apply Le Chatelier’s Principle to predict how changes in concentration, pressure, or temperature will affect the equilibrium of a reaction. Students are often tasked with calculating equilibrium concentrations or partial pressures. To approach this question, students should:
- Write the equilibrium expression and calculate the equilibrium constant (K).
- Predict the shift in equilibrium position based on changes in temperature or pressure.
- Use the reaction quotient (Q) to compare with the equilibrium constant and determine the direction of the reaction shift.
By breaking down each question in detail, students can target their studying efforts more effectively, ensuring they understand the core concepts needed to approach each section with confidence.
Scoring Criteria for Free Response Answers
This section explains the key factors that contribute to the evaluation of written responses during the assessment. Understanding the scoring rubric allows students to tailor their answers in a way that maximizes their performance. The criteria focus not only on accuracy but also on clarity, completeness, and logical reasoning.
Key Elements of Scoring
Each response is graded based on several core components that reflect the depth of understanding and the quality of the response. These components include:
- Accuracy: The correctness of the information presented. Responses that contain errors or omissions in key facts typically receive lower scores.
- Clarity and Organization: How clearly and logically the answer is structured. A well-organized response is easier to follow and demonstrates a more coherent thought process.
- Relevance: The extent to which the response addresses the question asked. Irrelevant or off-topic information can result in point deductions.
- Completeness: Whether the response covers all aspects of the question, including all necessary calculations, explanations, or examples.
- Justification: Providing adequate explanations or reasoning to support answers. Responses should demonstrate how conclusions are reached, especially in problem-solving or conceptual questions.
Scoring Rubric Overview
Each question is typically divided into parts, with points allocated to specific aspects of the response. The overall score reflects how well the student addresses each part of the question, using the following guidelines:
- 1-Point Criterion: Basic knowledge or simple calculations. These are often straightforward answers that require minimal explanation.
- 2-Point Criterion: Intermediate steps or intermediate-level reasoning. The response might involve some calculation or conceptual explanation with fewer details than higher-level criteria.
- 3-Point Criterion: Detailed reasoning or multi-step problem-solving. This includes comprehensive answers where students must demonstrate deeper understanding, with clear explanations and justification of their steps.
- 4-Point Criterion: High-level responses that require advanced understanding. These responses typically include complex problem-solving, in-depth explanations, and a clear demonstration of conceptual mastery.
In conclusion, achieving high scores on written sections requires a balance of accuracy, clarity, and depth. Practicing writing responses that address all parts of a question and explaining the reasoning behind each step is crucial for success.
How to Interpret the Grading Rubric
Understanding how to read and interpret the grading rubric is essential for any exam. The rubric serves as a guide for both students and graders, ensuring that responses are evaluated consistently based on specific criteria. Students can use the rubric to focus their efforts on what the exam expects, while also gaining insight into how to structure their answers for maximum effectiveness.
Breaking Down the Rubric
The grading rubric typically includes several components that align with the most important aspects of the assessment. Each section of the rubric is designed to evaluate specific skills and content knowledge. The general criteria include:
- Accuracy: This measures the correctness of the information presented. A high score depends on demonstrating the right facts, formulas, or principles clearly and without error.
- Organization: How well the answer is structured. A clear, logically organized response that is easy to follow tends to score higher. Proper use of paragraphs, headings, and numbered steps is often encouraged.
- Completeness: This criterion ensures that all aspects of the question are addressed. Missing parts of the answer, such as calculations or necessary explanations, can lead to point deductions.
- Clarity of Explanation: It is not enough to simply present the correct information. The reasoning behind the solution must be explained thoroughly. Well-articulated steps demonstrate understanding and score higher than bare-bones answers.
How to Maximize Your Score Using the Rubric
To effectively interpret and use the rubric, consider these strategies:
- Understand Key Points: Focus on the components of the rubric that align with the most heavily weighted aspects of the question. For instance, if a question requires both calculations and an explanation, make sure to balance both accurately and comprehensively.
- Explain Your Thought Process: Even if the solution is correct, a well-supported explanation helps in demonstrating depth of understanding. Explain each step, particularly in multi-step problems.
- Review the Rubric After the Exam: Once you finish answering, review the rubric and double-check if you have fully met the listed criteria. This can ensure you didn’t overlook any important part of the question.
In summary, interpreting the grading rubric allows students to focus on the specific areas that matter most for scoring well. A careful, detailed approach that addresses every component in the rubric can significantly improve the likelihood of achieving a high score.
Essential Chemistry Topics for the Exam
When preparing for an advanced scientific examination, it is crucial to focus on the key concepts and areas that are most frequently tested. A solid understanding of these core topics not only helps in tackling various problems effectively but also ensures that you are well-prepared for a wide range of questions. This section highlights the fundamental subjects that you should prioritize in your study plan to perform well in the test.
Core Concepts in Chemical Reactions
The ability to understand and predict chemical reactions is essential for excelling in the examination. Some of the most important concepts in this area include:
- Stoichiometry: The calculation of reactants and products in chemical reactions, using molar ratios and balancing equations.
- Thermodynamics: Understanding the principles of energy transfer, enthalpy, and the laws of thermodynamics as they relate to chemical reactions.
- Kinetics: The study of reaction rates and the factors that influence them, such as concentration, temperature, and catalysts.
Key Principles in Chemical Bonding
Understanding the nature of bonds and how atoms interact to form molecules is another crucial area of focus. Key topics include:
- Covalent and Ionic Bonds: The distinction between bonds formed by electron sharing and electron transfer.
- Electronegativity: The tendency of atoms to attract electrons and how it influences the nature of bonds.
- Molecular Geometry: The shapes of molecules based on electron pair repulsion, as predicted by the VSEPR theory.
Focusing on these essential topics will ensure that you are equipped with the knowledge needed to solve a variety of problems. A well-rounded understanding of these areas will not only help with multiple-choice questions but also provide a solid foundation for tackling more complex scenarios in open-ended portions of the exam.
Reviewing Stoichiometry in Free Response
Stoichiometry is a fundamental topic that frequently appears in open-ended sections of scientific assessments. It involves the calculation of quantities in chemical reactions, based on the relationships between reactants and products. Mastering stoichiometry is crucial, as it enables you to solve problems involving mass, volume, or moles of substances, and apply these concepts to a variety of experimental scenarios.
When tackling stoichiometry in the test, it is important to approach the questions systematically. First, make sure you understand the balanced equation for the reaction, as it provides the necessary molar ratios. From there, you can determine how the quantities of one substance relate to another, using dimensional analysis or conversion factors. Here are some key elements to keep in mind:
- Balancing Equations: Always ensure that the chemical equation is correctly balanced. This provides the molar ratios needed for accurate calculations.
- Unit Conversions: Convert between units such as moles, grams, liters, and molecules using the appropriate conversion factors.
- Limiting Reactants: Be prepared to identify limiting reactants in a reaction, which determines the maximum amount of product that can be formed.
- Excess Reactants: Understand how to calculate the remaining amount of excess reactants after the reaction is complete.
By focusing on these key steps, you can approach stoichiometry questions with confidence, ensuring accurate calculations and clear explanations. A strong grasp of stoichiometric principles not only helps you solve problems efficiently but also strengthens your overall understanding of chemical processes.
| Step | Action |
|---|---|
| 1 | Write and balance the chemical equation |
| 2 | Identify known quantities and the unknown variable |
| 3 | Use stoichiometric ratios to convert between units |
| 4 | Account for limiting and excess reactants |
| 5 | Double-check units and calculations |
With these steps in mind, you can confidently approach stoichiometry problems, ensuring accuracy and clarity in your responses. Proper preparation will allow you to handle any related questions effectively, even when they present complex or multi-step calculations.
Understanding Thermodynamics Questions
Thermodynamics plays a crucial role in understanding how energy behaves within chemical systems. When tackling questions on this topic, it’s essential to focus on the principles that govern the transfer and transformation of energy. Whether you’re asked about heat, work, or the efficiency of a process, grasping the core concepts can help you analyze and interpret the given scenarios accurately.
The key to answering thermodynamics-related questions effectively lies in recognizing the fundamental laws and equations involved. For instance, knowing how to apply the first law of thermodynamics, which deals with the conservation of energy, or understanding the concept of entropy, which reflects disorder or randomness, can make a significant difference in solving problems.
Common Thermodynamic Concepts to Review
- Internal Energy: This refers to the total energy contained within a system, including kinetic and potential energy of the molecules.
- Enthalpy: The heat content of a system, useful for studying processes at constant pressure, like chemical reactions or phase changes.
- Entropy: A measure of disorder or randomness in a system, which tends to increase in spontaneous processes.
- Gibbs Free Energy: A critical function for determining whether a reaction will occur spontaneously, combining both enthalpy and entropy considerations.
Strategies for Success
- Carefully analyze the question: Focus on the specific conditions provided, such as temperature, pressure, and the phase of the substance.
- Identify the appropriate equation: Use the correct thermodynamic equation based on the type of problem you’re solving, whether it’s for work, heat, or changes in energy.
- Pay attention to units: Thermodynamic calculations often require careful unit conversions. Ensure consistency in units to avoid errors.
- Check spontaneity: If you’re asked about the spontaneity of a process, use Gibbs free energy and examine whether it is negative, indicating a spontaneous reaction.
By focusing on these core ideas and reviewing the relevant formulas, you’ll be prepared to handle thermodynamics questions with confidence. Understanding these key concepts allows for a structured approach, making complex problems easier to solve and ensuring your answers are accurate and well-explained.
Organic Chemistry in Free Response Questions
Organic compounds and their reactions are a significant aspect of any examination related to molecular transformations. These questions test your understanding of functional groups, reaction mechanisms, and the ability to synthesize complex molecules. Mastery of key organic reactions and the ability to identify reaction types quickly can help in breaking down and solving complex scenarios presented during the exam.
The focus of organic chemistry questions often involves interpreting reaction schemes, predicting products, and understanding how various reagents influence the outcome of chemical processes. It’s important to approach these problems systematically, ensuring that you not only recall reactions but also apply the appropriate steps in a logical manner. Familiarity with mechanisms such as electrophilic addition, nucleophilic substitution, and elimination reactions is crucial for success.
Key Organic Concepts to Focus On
- Functional Groups: Recognizing common functional groups such as alcohols, aldehydes, ketones, and carboxylic acids is critical for identifying reactivity and expected products.
- Reaction Mechanisms: Understanding the flow of electrons in reactions like electrophilic addition, nucleophilic substitution, and rearrangements helps in predicting products and intermediates.
- Stereochemistry: Be prepared to explain chirality, optical activity, and the difference between enantiomers and diastereomers.
- Synthetic Strategies: Knowledge of how to construct molecules from simpler precursors and understanding how to manipulate functional groups in multi-step reactions are essential for more complex problems.
Tips for Tackling Organic Questions
- Practice mechanisms: Familiarize yourself with how different organic reactions proceed and the order in which bonds are formed or broken.
- Draw structures: Visualizing molecules and their intermediates helps in better understanding reaction pathways and predicting products more accurately.
- Understand regioselectivity and stereoselectivity: Many organic reactions yield specific products depending on the position or spatial arrangement of atoms in the molecule.
- Know common reagents: Recognizing reagents and their roles in promoting certain reactions, such as oxidation, reduction, or substitution, can streamline your approach to problems.
| Reaction Type | Example Reaction | Common Reagents |
|---|---|---|
| Electrophilic Addition | Alkene + HBr → Alkyl Bromide | HBr, HCl |
| Nucleophilic Substitution | R-Br + OH- → R-OH + Br- | OH-, NaOH |
| Elimination | Alkyl Halide → Alkene + HX | KOH, NaOEt |
Organic chemistry questions require not only knowledge of the reactions but also the ability to apply them to different types of molecules. By reviewing the foundational principles and practicing problem-solving strategies, you can confidently approach and solve these questions effectively.
Resources for Further AP Chemistry Study
To excel in any high-level science examination, it’s crucial to have access to diverse materials that help reinforce key concepts and provide practice opportunities. A variety of study tools, including textbooks, online platforms, and practice exams, can help you refine your understanding and improve your performance. Building a solid foundation with quality resources will enable you to approach complex questions with confidence and precision.
Essential Textbooks and Study Guides
- Textbooks: Refer to comprehensive texts that explain fundamental principles in detail. Some popular choices include Principles of Modern Chemistry and Chemistry: The Central Science. These textbooks offer clear explanations and practice problems to test your knowledge.
- Study Guides: Guides such as the AP Chemistry Crash Course and Barron’s AP Chemistry provide condensed versions of key topics, along with exam strategies and practice questions tailored for the exam format.
- Workbooks: Practice workbooks are invaluable for self-testing. These resources often include full-length practice exams and step-by-step solutions that can help clarify difficult concepts.
Online Resources and Platforms
- Khan Academy: An excellent, free online resource that offers video lessons on a wide range of topics. These videos break down complex concepts into manageable segments, making them accessible for all learners.
- AP Classroom: This official resource from the College Board offers practice exams, progress tracking, and personalized feedback. It’s an essential tool for understanding your strengths and weaknesses.
- Chegg Study: Known for its homework help, Chegg also provides access to practice problems and detailed explanations, which can assist in mastering difficult topics.
Practice Exams and Flashcards
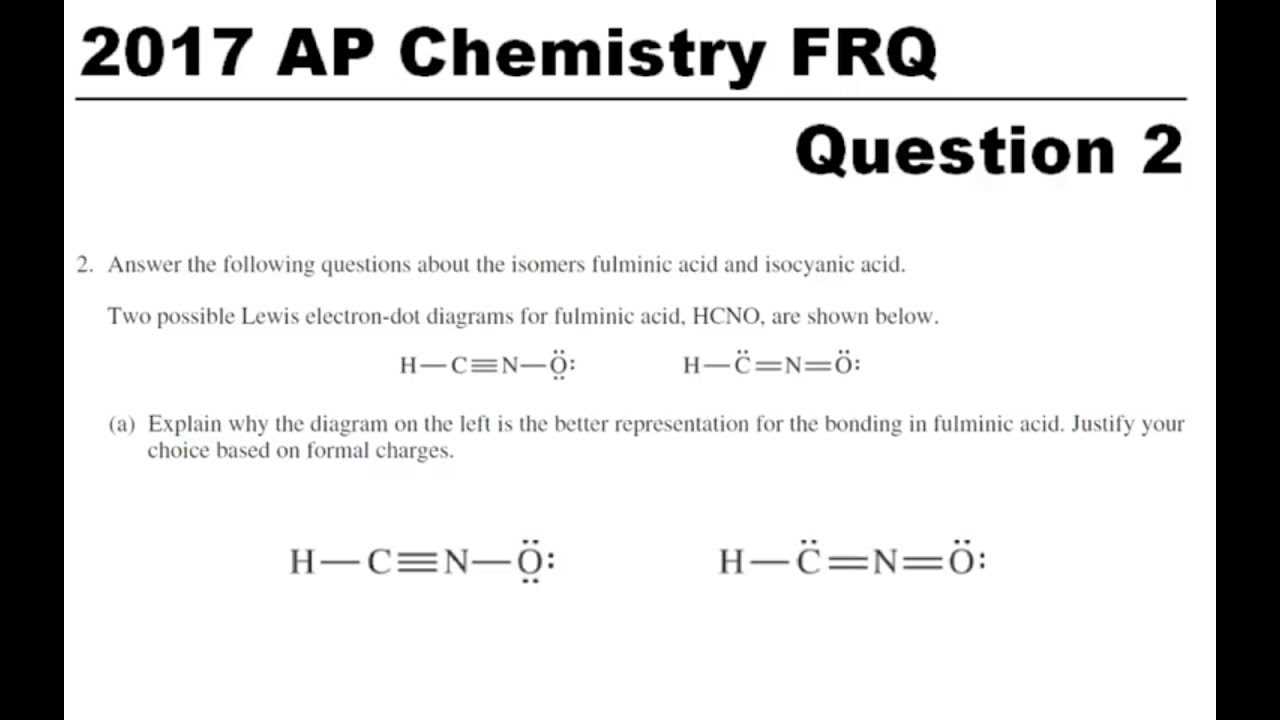
- Previous Exams: Access past exams to familiarize yourself with the format and types of questions you’ll encounter. Practicing under timed conditions can also improve your test-taking strategies.
- Flashcards: Create or use online flashcards to reinforce key terms, formulas, and reaction mechanisms. Platforms like Quizlet allow you to access pre-made flashcards and create your own personalized study sets.
Utilizing these resources will help you identify your weak areas, reinforce your strengths, and ultimately ensure that you are well-prepared for any challenging assessment. Stay consistent with your practice and leverage the wide array of materials available to boost your confidence and knowledge.