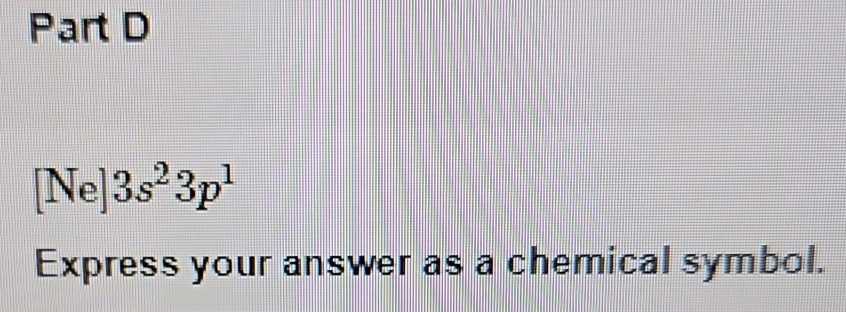
How to Express Your Answer as a Chemical Symbol
In scientific disciplines, simplifying complex concepts often involves using concise representations. This approach allows for faster communication and clearer understanding, especially when dealing with intricate data or reactions. By mastering these shorthand methods, anyone can navigate through vast amounts of information with ease and precision.
Symbols play a crucial role in this process, acting as compact forms of knowledge. Whether referring to elements in the periodic table or denoting specific properties, these shorthand characters hold great significance in the world of science. Understanding how to use these notations accurately is essential for interpreting and communicating scientific ideas.
As science continues to evolve, the language of symbols becomes even more fundamental. The ability to decode and apply these notations opens doors to a deeper understanding of the natural world, enabling clear explanations of complex theories and reactions. In this section, we explore how to interpret and utilize these representations effectively in various contexts.
Understanding Chemical Symbols and Their Usage
In the realm of science, shorthand notations are used to represent complex ideas in a simplified form. These notations are designed to convey a wealth of information with just a few characters, making it easier for scientists and researchers to communicate complex concepts quickly and accurately. Mastering this form of expression is essential for anyone looking to delve deeper into scientific fields such as chemistry and physics.
The Role of Abbreviations in Science
These notations, often based on the periodic table or molecular structures, are fundamental to understanding the building blocks of matter. They are used across various scientific disciplines to streamline discussions, calculations, and documentation. Without these concise representations, the complexity of scientific work would be overwhelming, making it difficult to exchange and interpret ideas efficiently.
Common Examples of Notations
- Elemental Symbols: Represent the basic units of matter, such as “H” for hydrogen or “O” for oxygen.
- Atomic Numbers: Indicate the number of protons in an atom’s nucleus, crucial for identifying elements.
- Molecular Formulas: Provide information about the composition of compounds, like “H2O” for water.
- Subscripts and Superscripts: Show the number of atoms or ions in a molecule and their charges.
Understanding these notations is not just about memorizing symbols but also about recognizing their importance in conveying scientific meaning. By learning how to interpret and use them correctly, individuals can navigate the complexities of scientific literature and experimental data with greater ease.
The Basics of Chemical Notation
Scientific notations are essential tools used to represent elements, compounds, and reactions in a concise and understandable way. They allow scientists to convey complex information in a simplified form, making it easier to work with and share data. Learning the basics of this shorthand method is crucial for anyone involved in the study of matter and its interactions.
Elemental Representation
At the foundation of scientific notation are the representations of elements. Each element is assigned a unique set of letters, usually derived from its Latin name, which makes it universally recognizable. For example, “Na” represents sodium, derived from “natrium,” its Latin name. These notations allow for quick identification and avoid confusion when discussing multiple substances.
Compound Formulas
Beyond individual elements, notations are also used to depict the combinations of elements that form compounds. These combinations are written using the elemental symbols of the atoms involved, along with subscripts to indicate the number of atoms. For example, “CO2” represents carbon dioxide, where the subscript “2” indicates two oxygen atoms bonded to one carbon atom. This format helps in understanding the stoichiometry of chemical reactions and molecular structures.
Importance of Chemical Symbols in Chemistry
In the field of chemistry, shorthand notations are crucial for effective communication and understanding. These notations allow scientists to represent complex substances and reactions in a way that is both efficient and precise. Without these concise forms, conveying intricate details about compounds, reactions, and atomic structures would be much more difficult and time-consuming.
Facilitating Clear Communication
One of the primary roles of these notations is to streamline communication. By using universally accepted shorthand, chemists and researchers can exchange ideas without confusion. This is especially important when dealing with international collaborations or when explaining intricate concepts in academic literature.
- Universality: Notations ensure that all scientists, regardless of their native language, can understand and use the same terms.
- Efficiency: Instead of writing out full names of compounds or elements, shorthand allows for quicker and clearer notation.
- Precision: The ability to accurately depict the atomic structure and composition of substances eliminates ambiguity in scientific discussions.
Understanding Complex Reactions
These notations are also vital in understanding how different substances interact in chemical reactions. By using simple representations, it’s easier to visualize the changes occurring at the molecular level. For example, the notation for water, “H2O,” immediately tells us the ratio of hydrogen to oxygen in the molecule, helping to explain its behavior in various reactions.
How to Write Chemical Formulas Correctly
Writing formulas accurately is essential for conveying the right information about the composition of a substance. These notations help chemists to easily understand the elements involved and their respective ratios. Properly writing these representations ensures clarity and prevents confusion in scientific communication, especially when discussing reactions or molecular properties.
To write formulas correctly, it’s important to follow specific rules that govern the placement of element symbols, numerical subscripts, and other related notations. Understanding these guidelines will enable you to represent compounds and molecules with precision.
| Rule | Example |
|---|---|
| Element symbols must be written with the first letter capitalized and the second letter (if any) lowercase. | H for hydrogen, O for oxygen |
| Subscripts indicate the number of atoms of an element in a molecule. | H2O for water (2 hydrogen atoms and 1 oxygen atom) |
| When more than one element is involved, their symbols are written together. | CO2 for carbon dioxide |
| For ionic compounds, the ratio of ions is balanced based on charge. | NaCl for sodium chloride (1 sodium ion and 1 chloride ion) |
By following these basic rules, anyone can create accurate notations for various substances. This skill is fundamental in all branches of chemistry, from inorganic chemistry to biochemistry, and is critical for understanding the structure and behavior of matter.
The Role of Atomic Number in Symbols
The atomic number plays a fundamental role in the identification and classification of elements. It determines the number of protons found in the nucleus of an atom and uniquely identifies each element in the periodic table. This number is essential not only for understanding the structure of an element but also for interpreting its chemical properties and behavior in various reactions.
In notations, the atomic number is often represented as a subscript before the element’s symbol, providing key information that distinguishes one element from another. This simple yet powerful representation allows chemists to quickly identify an element’s position on the periodic table and understand its characteristics based on its atomic number.
For example, the element hydrogen has an atomic number of 1, which is reflected in its symbol as “H”. The atomic number directly correlates to its position in the table and gives insight into its reactivity and interactions with other elements. Understanding the role of the atomic number is essential for correctly interpreting and using element symbols in scientific discussions.
Common Mistakes in Using Chemical Symbols
Inaccurate use of notations can lead to confusion and misunderstanding in scientific discussions. Even small mistakes in representing elements or compounds can result in incorrect conclusions, making it important to be aware of common errors. These mistakes often arise from misinterpretation of rules or lack of attention to detail, which can have significant consequences in both academic and practical applications.
One frequent mistake is capitalizing the second letter of an element’s abbreviation. For instance, “Co” (for cobalt) should not be written as “CO”, which could confuse it with carbon monoxide. The correct form is crucial for distinguishing between different substances and ensuring clarity in scientific communication.
Another common error involves incorrect use of subscripts. Subscripts indicate the number of atoms in a molecule, but sometimes they are omitted or miswritten. For example, writing “H2O” as “HO” would be misleading, as it omits one hydrogen atom and changes the meaning of the substance entirely. Proper use of these numerical indicators is essential for conveying accurate information about molecular structures.
These errors can easily be avoided by following standardized conventions and paying attention to the specific rules for notation. Understanding and practicing these guidelines is key to ensuring accuracy and preventing misunderstandings in scientific work.
How Chemical Symbols Relate to Elements
Each substance in the universe is made up of fundamental building blocks, and each of these building blocks is represented by a unique shorthand. These notations serve as identifiers for various materials found in nature and are used to simplify complex information. The relationship between these notations and the elements they represent is essential for organizing and understanding the vast number of substances around us.
The notations for elements are universally accepted, allowing scientists from all over the world to communicate with ease. These representations are based on the Latin names or other historical sources, providing a consistent method for identifying the elements in a standardized way. Here’s how these abbreviations relate directly to elements:
- Unique Representation: Every element has a specific abbreviation, making it easily identifiable in scientific discussions. For instance, “H” stands for hydrogen, and “O” stands for oxygen.
- Consistent Naming Convention: The abbreviations are based on the element’s name in English or its Latin roots. For example, “Na” for sodium comes from its Latin name “Natrium.”
- Periodic Table Reference: These notations are organized within the periodic table, with each element’s abbreviation reflecting its position. Elements in the same group or period often have similar properties, which can be inferred from their notations.
By using these consistent notations, scientists are able to communicate the structure, properties, and behavior of elements in a way that is easy to understand and universally recognized, regardless of language barriers or local naming conventions.
Reading and Interpreting Chemical Equations
Understanding the way substances interact and transform during reactions is a core principle in science. The representation of these processes through specific notations helps to simplify complex ideas and ensures clarity in communication. Interpreting these expressions correctly allows scientists to predict outcomes and understand the fundamental principles governing reactions.
A well-structured equation shows how reactants combine or break apart to form products. The notation used conveys not only the substances involved but also the proportions and the conservation of mass. Properly reading these equations requires an understanding of both the elements and the relationships between them.
- Reactants and Products: The substances on the left side of the equation are the starting materials, while those on the right represent the products formed.
- Coefficients: Numbers placed before a substance indicate the quantity of molecules or atoms involved in the reaction, ensuring that the equation balances.
- Law of Conservation of Mass: The total number of atoms for each element must be the same on both sides of the equation, reflecting the principle that matter is neither created nor destroyed.
By practicing how to read and interpret these expressions, one can better understand the mechanisms of reactions and the specific roles of different substances in those processes.
The Significance of Elemental Abbreviations
In scientific communication, brevity and clarity are essential, especially when dealing with the vast number of substances. Abbreviations for elements provide a standardized and efficient way to convey complex information. These short notations allow for quick identification, ease of use, and consistency across various fields of study.
These abbreviations, often derived from Latin or the element’s common name, are universally recognized and used across the globe. This global standardization eliminates the potential for confusion, enabling scientists from different regions to collaborate effectively. Whether discussing molecular structures, chemical reactions, or physical properties, the use of elemental abbreviations is vital for clear and precise communication.
Efficiency in Scientific Communication
Elemental abbreviations save time and space in both written and verbal exchanges. Instead of writing out full names of substances, the short notations allow researchers to convey complex concepts quickly and with fewer chances for error. This is especially important when dealing with lengthy reactions, formulas, and processes.
Consistency and Universality
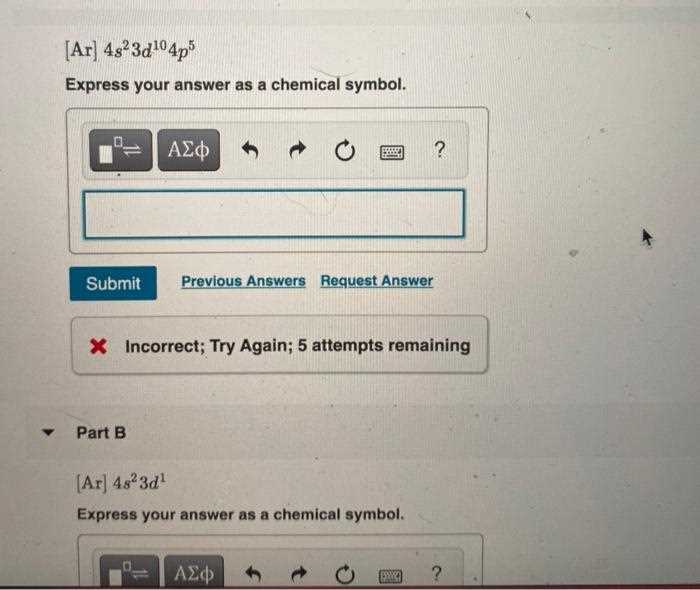
These abbreviations provide a universal language for scientists worldwide. The consistency of elemental abbreviations ensures that no matter where research is conducted, the meaning remains the same. For example, “Na” always refers to sodium, whether the research takes place in North America, Europe, or Asia.
Understanding the importance of these abbreviations helps scientists navigate the world of materials and compounds with ease, ensuring accurate representation and communication in all areas of science.
Using Subscripts and Superscripts in Chemistry
In the field of science, especially when discussing molecular structures and reactions, precise notation is essential for conveying accurate information. Subscripts and superscripts play a crucial role in these notations, helping to represent the quantity of atoms, charge, or other important details in a compact and easily understandable format. These small but powerful markers are integral to both chemical formulas and equations.
Subscripts are commonly used to indicate the number of atoms of an element in a molecule, while superscripts are employed to show the charge of ions or the number of isotopes. Proper use of these indicators is essential for accurately interpreting and writing formulas, ensuring that the molecular structure is clearly communicated and understood.
Subscripts in Molecular Formulas
Subscripts indicate how many atoms of each element are present in a compound. They appear after the element’s abbreviation and are placed lower than the main line of text. For example, in water (H2O), the subscript “2” tells you there are two hydrogen atoms for each oxygen atom in the molecule.
| Subscript Example | Meaning |
|---|---|
| H2O | Two hydrogen atoms and one oxygen atom |
| C6H12O6 | Six carbon atoms, twelve hydrogen atoms, and six oxygen atoms |
Superscripts in Ionic Compounds
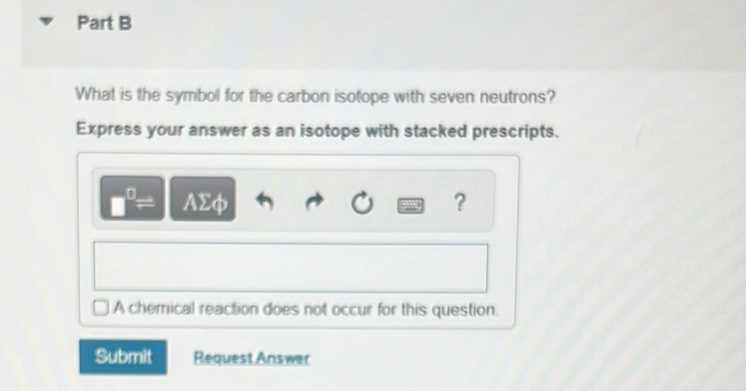
Superscripts are used to represent the charge of ions or the number of electrons gained or lost by an atom. When an element forms an ion, the superscript indicates whether the ion has a positive or negative charge and the magnitude of that charge. For example, Na+ indicates a sodium ion with a single positive charge, while Cl– denotes a chloride ion with a negative charge.
| Superscript Example | Meaning |
|---|---|
| Na+ | Sodium ion with a +1 charge |
| Cl– | Chloride ion with a -1 charge |
Understanding the use of subscripts and superscripts is essential for interpreting molecular structures, balancing equations, and ensuring the accuracy of scientific work. These notations allow chemists to convey complex information in a simple and standardized way, making them indispensable tools in the study of matter.
Common Chemical Symbols and Their Meanings
In the world of science, there are certain notations that are universally recognized and used to represent elements and compounds. These shorthand representations are critical for simplifying complex concepts, making it easier for scientists to communicate about materials, reactions, and molecular structures. Understanding these common notations is key to working with substances in both laboratory and theoretical settings.
The notations used for elements are typically derived from their Latin names or commonly used English terms. Each one provides essential information about the substance, from its atomic structure to its behavior in various reactions. Recognizing these abbreviations allows scientists to quickly interpret and apply the necessary information.
Frequently Used Elemental Abbreviations
Here are some of the most common elemental abbreviations and their meanings:
- H: Hydrogen, the simplest and most abundant element in the universe.
- O: Oxygen, essential for respiration and combustion.
- C: Carbon, a building block of life, present in all organic compounds.
- Na: Sodium, a highly reactive alkali metal commonly found in salt.
- Cl: Chlorine, a halogen often used in disinfectants and bleaching agents.
- Fe: Iron, a key element in blood and a component of many alloys.
Understanding Elemental Abbreviations in Reactions
These abbreviations play a crucial role when expressing reactions between substances. For instance, in the equation 2H2 + O2 → 2H2O, the notation “H” represents hydrogen, and “O” stands for oxygen. Recognizing and interpreting these symbols properly is essential for understanding the underlying processes in chemical interactions.
By mastering these commonly used notations, anyone working with substances or studying reactions can gain a clearer and more efficient understanding of the material at hand, ensuring greater accuracy and clarity in all areas of science.
How to Express Molecular Structures
When studying molecules, it is essential to represent their structures clearly and precisely. This allows scientists and students to visualize how atoms are bonded together and how they interact with each other. Proper notation and visualization methods are vital for understanding molecular behavior, reactivity, and properties.
There are various ways to convey the structure of a molecule, depending on the level of detail needed and the context in which it is used. Some representations focus on the connectivity between atoms, while others may include more detailed information about the angles, bonds, and spatial arrangement.
Structural Formulas
One of the most common ways to depict molecular structures is through structural formulas. These formulas show how atoms are connected within a molecule, often with lines representing bonds between atoms. For example, the structural formula for water (H2O) illustrates the bonding between hydrogen and oxygen atoms, with a line indicating the covalent bond.
Ball-and-Stick Models
For a more three-dimensional representation, ball-and-stick models are commonly used. These models show atoms as spheres (balls) and the bonds as sticks connecting them. This representation provides a clearer view of the molecular geometry, such as bond angles and overall shape. While not always to scale, these models are particularly useful for understanding complex molecules and visualizing how atoms are arranged in space.
Accurately representing molecular structures, whether through formulas or 3D models, is crucial for chemical analysis, designing new compounds, and predicting molecular behavior. By mastering these methods, one can gain a deeper understanding of the intricate world of molecules and their interactions.
Understanding Ion Symbols and Charges
In the study of matter, it is essential to understand how atoms can gain or lose electrons, resulting in charged species. These charged particles, called ions, play a crucial role in various chemical reactions, including those in biological systems and industrial processes. To represent ions accurately, specific notations and charges are used to reflect their nature and behavior.
Each ion is identified by its element and the number of electrons it has gained or lost. Positive ions, known as cations, occur when an atom loses electrons, while negative ions, or anions, form when electrons are gained. The notation for ions typically includes the elemental symbol followed by the charge, indicated by a superscript. Understanding these notations helps to interpret reactions and predict how ions will interact with other substances.
Cations and Anions
Cations are positively charged and are usually metals that lose electrons to achieve a stable electron configuration. For instance, sodium (Na) loses one electron to form Na+, while calcium (Ca) can lose two electrons to form Ca2+.
- Na+: Sodium ion, formed by the loss of one electron.
- Ca2+: Calcium ion, formed by the loss of two electrons.
Anions, on the other hand, are negatively charged ions formed when atoms gain electrons. Nonmetals typically form anions, such as chlorine (Cl) gaining one electron to form Cl– or oxygen (O) forming O2- after gaining two electrons.
Notating Ions in Reactions
The way ions are notated is vital in chemical equations, as it shows their role in reactions. For example, in a reaction between sodium and chlorine to form sodium chloride, the ions involved are Na+ and Cl–. These notations help clarify the electron transfer process, allowing scientists to better understand how compounds are formed and how ions interact in different environments.
Tips for Memorizing Chemical Symbols
Learning the shorthand representations of elements can seem like a daunting task due to the large number of substances in nature. However, with the right strategies, remembering these notations can become much easier. The key is to find methods that work for individual learning styles, whether through association, repetition, or categorization.
One effective approach is to group elements by their properties or periodic table families. This can help in remembering which elements share similar characteristics, making it easier to recall their notations when needed. Another helpful technique is to use mnemonics or visual aids to associate the symbol with the element’s name or its common uses. For example, remembering that “H” stands for hydrogen, which is the most abundant element in the universe, can reinforce its symbol in the mind.
Mnemonic Devices and Associations
Mnemonics can play a huge role in simplifying the learning process. For example, to remember the first few elements on the periodic table, you might create a sentence such as “Happy Harry Hates Being Bored,” representing H (Hydrogen), He (Helium), Li (Lithium), Be (Beryllium), and B (Boron). These sentences make it easier to remember the sequence of elements and their corresponding notations.
Flashcards and Repetition
Another helpful tool is the use of flashcards. Write the name of the element on one side and its abbreviation on the other. Regularly reviewing these cards can help reinforce the associations between the names and their shorthand forms. With consistent repetition, recognition of these notations will become automatic, making it easier to recall them during discussions or exams.
| Element | Symbol | Mnemonic |
|---|---|---|
| Hydrogen | H | Happy |
| Helium | He | Harry |
| Lithium | Li | Hates |
| Beryllium | Be | Being |
| Boron | B | Bored |
With these techniques, memorizing the notations of elements becomes a much less challenging task, and soon the shorthand forms will become second nature.
Symbolism in Organic Chemistry
In the realm of organic science, visual representations are essential for conveying complex molecular structures and reactions in a concise and understandable way. The use of shorthand notations allows scientists to communicate intricate details about molecular bonds, functional groups, and reactions without the need for lengthy descriptions. These symbols not only save time but also provide a standardized method for interpreting the vast variety of organic compounds and their interactions.
Within this field, the use of specific notations plays a critical role in distinguishing between different types of compounds. For instance, elements such as carbon, hydrogen, and oxygen are typically represented by their respective abbreviations, while bonds between atoms are often depicted using lines or other symbols to indicate their strength and type. This system of representation enables chemists to visualize molecular structures quickly and efficiently.
Common Notations in Organic Reactions

In organic reactions, arrows are often used to indicate the movement of electrons or the direction of a reaction. A curved arrow typically represents the flow of electron pairs, while a straight arrow shows the transformation of reactants into products. These symbols are indispensable for illustrating reaction mechanisms and understanding how molecules change during a reaction.
Functional Groups and Their Notations
Functional groups, which are specific groups of atoms that impart certain properties to a molecule, also have distinct notations. For example, an alcohol group is denoted as -OH, while a carboxyl group is represented as -COOH. These symbols help in identifying the reactivity and chemical behavior of the compound at a glance, offering insight into its potential interactions in reactions.
Ultimately, the shorthand system used in organic chemistry allows for clarity, precision, and ease of communication, essential qualities when working with complex molecular structures and reactions.
The Link Between Atomic Mass and Symbols
The relationship between an element’s mass and its shorthand representation plays a crucial role in understanding its properties and behavior in various reactions. Atomic mass, which reflects the total number of protons and neutrons in an atom’s nucleus, is a fundamental characteristic that influences how elements interact with each other. While shorthand notations are used to represent these elements, it is the atomic mass that provides deeper insight into their composition and reactivity.
When an element is represented by its abbreviation, the number of protons, or the atomic number, is typically indicated. However, it is the atomic mass that often determines how that element behaves when forming compounds or undergoing reactions. In many cases, knowing the mass allows scientists to predict the stability of certain isotopes, which are variations of an element that have the same number of protons but a different number of neutrons.
Isotopes and Their Impact on Mass Notation
Isotopes are critical to the study of atomic mass because they represent different forms of the same element with distinct masses. For example, carbon exists as both carbon-12 and carbon-14, with the number indicating the total number of protons and neutrons. This distinction is important in fields like radiology, where the varying masses of isotopes lead to different behaviors in terms of radioactivity and half-life.
The Role of Atomic Mass in Chemical Reactions
The atomic mass of elements also affects how they combine to form compounds. During chemical reactions, the ratio of masses between reacting elements determines how molecules are formed. This concept is especially useful when balancing equations or calculating the quantities of reactants and products in a reaction. For instance, knowing the mass of an element allows for precise calculations of molar ratios, helping chemists achieve the desired outcome in a reaction.
In summary, atomic mass and its associated notation provide a vital link to understanding how elements and their variants behave in nature. It is through this connection that scientists can predict molecular behavior, optimize reactions, and explore new chemical pathways.
Practical Applications of Chemical Symbols
The shorthand notations used to represent elements and compounds are essential tools across various fields, simplifying the communication of complex information. These representations allow scientists, engineers, and professionals from diverse industries to convey intricate details about substances in a concise manner. Whether in research, manufacturing, or environmental science, these abbreviations are crucial for understanding materials and processes.
One of the most common areas where these notations are applied is in laboratory experiments, where precise measurement and identification of substances are vital. By using well-established conventions, professionals can ensure consistency in their work, enabling easy collaboration and reproducibility of results.
Applications in Research and Development
In scientific research, shorthand notations are indispensable when formulating hypotheses or presenting experimental data. By accurately representing elements and their compounds, researchers can clearly illustrate their findings without confusion. Some examples include:
- Representing molecular structures in organic chemistry to show how atoms are bonded.
- Identifying reactions in inorganic chemistry, such as precipitation or oxidation, through concise equations.
- Using isotopic notations to distinguish between different isotopes of an element for studies involving radiochemistry or nuclear physics.
Impact in Industry and Manufacturing
In the industrial sector, these notations are critical for ensuring the correct mixing, handling, and processing of materials. For example, in the production of alloys or pharmaceuticals, workers rely on these representations to confirm the exact elements or compounds involved in the manufacturing process. In industries like metallurgy, where the composition of materials affects their properties, knowing the correct abbreviations ensures that products meet specific standards. Applications include:
- Formulating precise chemical blends in industries like paints, coatings, and plastics.
- Creating standardized formulas for the production of high-quality medicines or dietary supplements.
- Tracking material compositions in aerospace engineering to maintain structural integrity and safety.
In conclusion, these shorthand notations are vital in various fields, from research to industry, enabling professionals to work efficiently and accurately. They streamline processes, improve communication, and ensure that complex information is easily understood and applied.