Complete Answers for Molecules of Life Worksheet

Understanding the fundamental elements that form the building blocks of organisms is crucial in biology. These core substances play a central role in processes that sustain life. From structural support to energy transfer, these key compounds drive essential cellular activities.
In this section, we will delve into the various types of organic substances found within living organisms. We’ll explore their structures, functions, and how they interact to maintain the intricate systems that make life possible. Topics will cover both simple and complex biomolecules, highlighting their diverse roles in growth, energy production, and genetic information storage.
By analyzing these compounds, we can gain insight into the mechanisms that support cellular processes. This understanding is essential not only for biology but also for advancing fields such as medicine and biotechnology. The interactions of these components offer a fascinating view into the complexity of life itself.
Molecules of Life Worksheet Answers
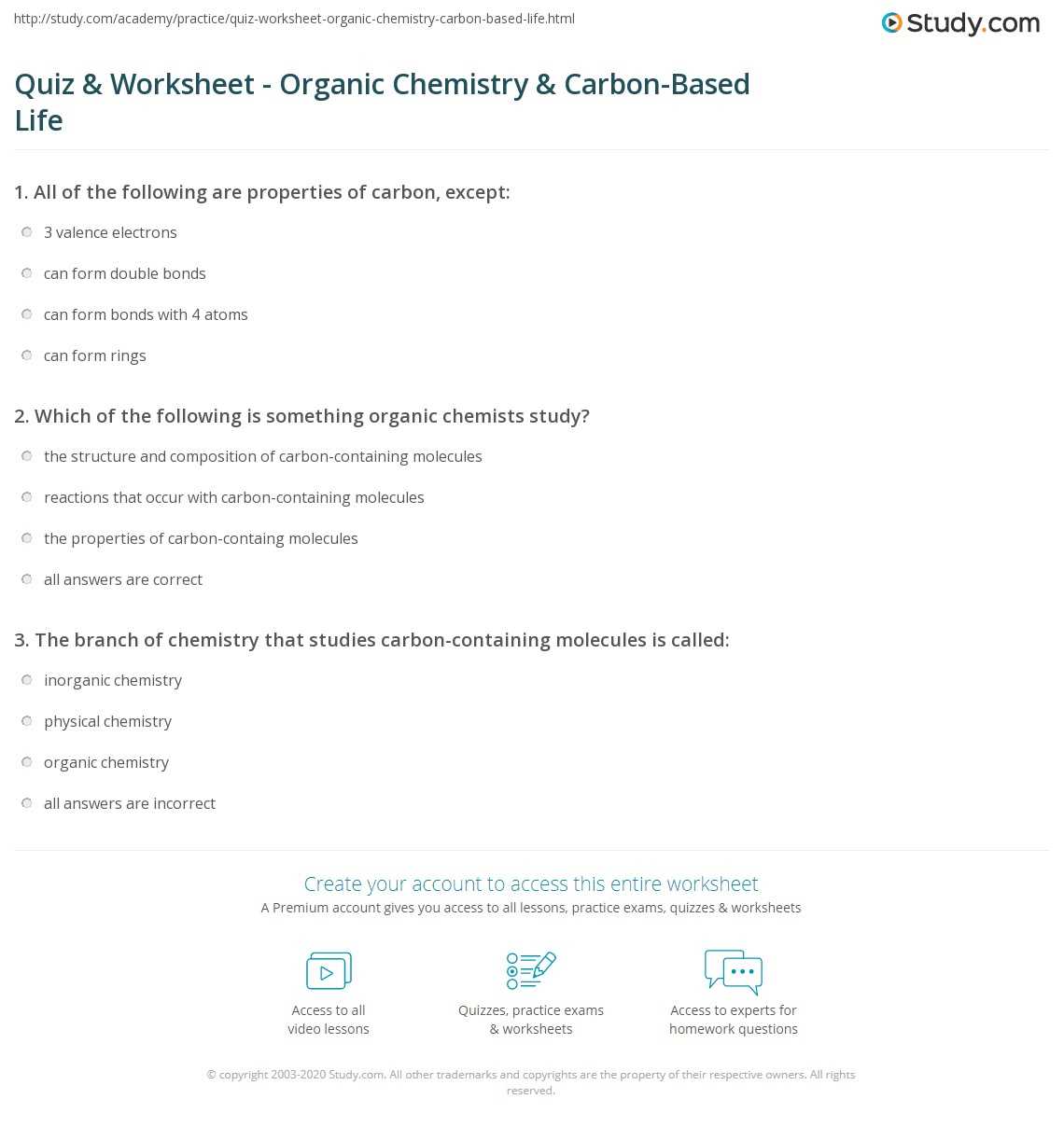
In this section, we will explore the core substances that are fundamental to the function of living organisms. These elements are essential in maintaining the processes that support growth, reproduction, and survival. By examining their structure and function, we gain a deeper understanding of how these compounds contribute to the overall system of biology.
Each of these compounds plays a specific role, working in harmony with others to ensure that organisms function properly. These compounds can be categorized into different groups, each with unique properties and functions.
- Proteins: Essential for building structures, enzymes, and facilitating cellular processes.
- Carbohydrates: The main energy source for cellular functions and metabolic activities.
- Lipids: Key components of cell membranes and energy storage molecules.
- Nucleic Acids: Carry genetic information and play a critical role in cell division and protein synthesis.
Each group interacts with others to form complex systems, enabling cells to perform the many tasks needed for survival. Through studying these interactions, we can understand the underlying mechanisms that keep organisms functioning efficiently.
By breaking down the functions of these compounds, students can gain a solid foundation in biological principles, which are vital for advanced studies in biology, genetics, and medicine. The knowledge of how these components interact provides valuable insights into the complexity of biological systems.
Understanding Biomolecules and Their Functions

Living organisms rely on a variety of essential substances that are crucial for the proper functioning of cells. These substances are involved in nearly every aspect of cellular processes, from energy production to the formation of structures. Understanding their roles and how they interact within biological systems is key to unraveling the complexities of life.
The major groups of these compounds each serve specific functions that are vital for the maintenance and growth of organisms. Some provide structural support, while others are involved in metabolic processes or storing and transmitting genetic information. These diverse functions illustrate the interconnected nature of cellular systems and the importance of these compounds in sustaining life.
Key Functions of Essential Compounds
Different types of these compounds perform unique and critical tasks within organisms. Some of the most important roles include:
- Energy storage: Compounds like carbohydrates and lipids serve as energy reserves for cells, providing fuel when needed.
- Cellular communication: Certain molecules act as signals, ensuring cells can communicate with each other and respond to their environment.
- Genetic information: Nucleic acids store and transmit genetic data, guiding the growth and reproduction of organisms.
- Structure and support: Proteins and lipids are integral in forming the structural components of cells, such as membranes and cytoskeletons.
The Interdependence of Biological Compounds
These compounds do not work in isolation; instead, they interact and depend on each other to support the functioning of the organism. For example, enzymes (a type of protein) facilitate reactions that are necessary for energy production, while lipids help to create the barriers that separate the inside of the cell from the external environment. Understanding these relationships is crucial for studying biology at a deeper level and recognizing how disruptions in these interactions can lead to disease.
Carbohydrates and Their Biological Roles
Carbohydrates are essential compounds that serve as a primary energy source for organisms. These substances are involved in various metabolic processes, providing both immediate and stored energy. They also play a crucial part in the structure and function of cells, tissues, and organs. Beyond energy, carbohydrates are central to many biological functions, influencing everything from cellular communication to immune responses.
Energy Supply: One of the main roles of carbohydrates is to supply energy. Through digestion and metabolic pathways, these compounds are broken down into glucose, a simple sugar that cells use to produce ATP, the energy currency of the body. The quick availability of glucose makes it a vital resource for active tissues, especially muscles and the brain.
Structural Functions: Carbohydrates contribute to the structural integrity of cells. In plants, for example, cellulose, a complex carbohydrate, forms the rigid cell walls, giving the plant its shape and support. In animals, certain carbohydrates are part of the extracellular matrix, which supports and organizes cells.
Cell Signaling and Recognition: Carbohydrates are involved in cell signaling and recognition. They are often attached to proteins and lipids on the cell surface, forming glycoproteins and glycolipids. These structures play key roles in immune response, allowing cells to identify pathogens, foreign substances, or damaged cells.
Storage of Energy: In addition to providing immediate energy, carbohydrates are also stored for later use. Animals store carbohydrates in the form of glycogen, primarily in the liver and muscles. When energy demands increase, glycogen is broken down into glucose to meet the body’s needs.
Lipids: Key to Cellular Membranes

Lipids are crucial components of cellular structures, playing a central role in forming protective barriers around cells. These substances contribute to the integrity and functionality of membranes, enabling them to control what enters and exits the cell. The unique properties of lipids, such as their hydrophobic nature, allow them to create effective barriers and organize cellular components.
In addition to forming membranes, lipids also participate in energy storage, signaling, and maintaining cellular health. Their versatility and structural diversity make them indispensable in the biochemical processes of all living organisms.
| Type of Lipid | Role in Membrane Structure |
|---|---|
| Phospholipids | Form the basic structure of the membrane, with hydrophilic heads facing outward and hydrophobic tails facing inward. |
| Cholesterol | Provides stability to the membrane, maintaining fluidity and preventing excessive rigidity or fluidity. |
| Glycolipids | Involved in cell recognition and communication, often attached to proteins on the membrane surface. |
Nucleic Acids and Genetic Information

Nucleic acids are fundamental to the storage and transmission of genetic information. They carry the instructions that guide cellular functions, development, and reproduction. These compounds are essential for the inheritance of traits from one generation to the next and for ensuring that cells operate according to their genetic blueprint. The sequence of building blocks within these substances encodes the information that shapes organisms at the molecular level.
The two main types of nucleic acids are involved in different processes: one stores the genetic instructions, while the other assists in the decoding and expression of that information. Together, they form the basis for cellular operations and biological diversity.
| Type of Nucleic Acid | Primary Function |
|---|---|
| DNA (Deoxyribonucleic Acid) | Stores genetic information and serves as a template for replication and protein synthesis. |
| RNA (Ribonucleic Acid) | Transfers genetic information from DNA and plays a role in protein synthesis and regulation of gene expression. |
Water as a Solvent in Biology
Water plays a crucial role in many biological processes due to its ability to dissolve a wide range of substances. Its unique properties make it an ideal medium for chemical reactions within living organisms. By dissolving salts, gases, and other compounds, water facilitates the transport of essential materials and the regulation of cellular functions. This solvent capability is vital for maintaining life’s biochemical processes.
Properties of Water as a Solvent
Water’s solvent properties are largely due to its polar nature. The uneven distribution of electrons in water molecules allows them to interact with various charged and polar substances. As a result, water can dissolve ionic compounds like salts and polar molecules like sugars, enabling them to move freely in solution. This ability is essential for nutrient transport, waste removal, and maintaining cellular structure.
Water’s Role in Biological Reactions
In addition to its solvent properties, water is also involved in many biochemical reactions, such as hydrolysis and condensation. These reactions are key to processes like digestion and the synthesis of complex molecules. By facilitating the breakdown of nutrients and the formation of new compounds, water supports metabolism and energy production within cells.
The Role of Amino Acids in Proteins
Amino acids are the fundamental building blocks that form proteins, which are essential for countless biological functions. The sequence and arrangement of these compounds determine the structure and function of proteins, influencing everything from enzyme activity to cellular repair. Each amino acid contributes specific properties that shape the protein’s final structure and its role within the body.
The diversity of amino acids, along with the way they interact with one another, allows proteins to carry out a vast array of functions. From acting as catalysts to supporting cellular structures, the importance of these compounds cannot be overstated.
| Amino Acid | Function in Proteins |
|---|---|
| Alanine | Plays a role in glucose metabolism and protein synthesis. |
| Glutamine | Important for immune system function and maintaining nitrogen balance. |
| Serine | Involved in the synthesis of nucleotides and lipids, as well as enzyme function. |
| Cysteine | Essential for the formation of disulfide bonds, contributing to protein structure stability. |
How Enzymes Catalyze Reactions
Enzymes play a crucial role in accelerating various biochemical processes within living organisms. These specialized proteins lower the energy required for chemical reactions to occur, enabling them to happen more quickly and efficiently. By binding to specific substances, enzymes facilitate the transformation of these compounds into new products. This process is vital for sustaining numerous physiological functions.
Enzymes exhibit remarkable specificity, meaning they only interact with particular substrates. Their function is determined by their unique structure, which allows them to recognize and bind to target molecules with high precision. Once the enzyme-substrate complex is formed, the reaction progresses more rapidly than it would without the enzyme’s involvement.
- Active Site: The region of the enzyme where the substrate binds and undergoes a chemical reaction.
- Substrate: The molecule that the enzyme acts upon to produce a specific result.
- Activation Energy: The energy required to initiate a reaction, which enzymes help reduce.
Through this catalytic activity, enzymes ensure that essential biochemical reactions occur under the right conditions and at the appropriate speeds, enabling organisms to maintain balance and carry out vital functions such as digestion, metabolism, and cell repair.
Identifying the Building Blocks of Life
At the core of every living organism are fundamental components that serve as the foundation for more complex structures and functions. These basic units interact to form the intricate networks that sustain life processes. Understanding these essential parts is key to deciphering how biological systems work and how they maintain their balance.
Key Elements and Their Roles
Every organism is made up of several key elements that combine in various ways to form the essential components of cells and tissues. Among the most critical elements are carbon, hydrogen, oxygen, and nitrogen. These atoms form the backbone of complex structures and are involved in nearly every biochemical reaction that occurs.
Primary Structural Units
The primary building blocks of organisms can be categorized into several types of substances, each serving a specific function:
- Amino Acids: The basic units of proteins, which are essential for nearly every biological process.
- Fatty Acids: Components of lipids, which provide energy storage and play a critical role in cell structure.
- Nucleotides: The building blocks of nucleic acids, which carry genetic information.
- Sugars: Simple carbohydrates that provide energy and contribute to cell structure.
These basic units not only form the structural framework of organisms but also enable the intricate biochemical reactions that are essential for growth, reproduction, and survival. Their combination and arrangement determine the specific characteristics and capabilities of living beings.
Cellular Respiration and Energy Production
The process by which cells generate the energy needed for vital functions is essential for maintaining all biological activities. Through a series of intricate biochemical reactions, cells break down nutrients to release energy, which is then stored in a form that can be easily utilized when needed. This energy production is a cornerstone for growth, repair, and everyday cellular processes.
Stages of Energy Production
Energy production occurs in multiple stages, each contributing to the efficient release and storage of energy:
- Glycolysis: The initial step that occurs in the cell’s cytoplasm, where glucose is broken down into smaller units, releasing a small amount of energy.
- Krebs Cycle: This cycle takes place in the mitochondria, where the breakdown products from glycolysis are further processed to generate energy-rich molecules.
- Electron Transport Chain: The final stage occurs in the mitochondria, where high-energy electrons are transferred to generate a significant amount of energy, stored as ATP (adenosine triphosphate).
Role of ATP in Cellular Function
ATP is the primary energy carrier in cells. It powers nearly every cellular process, from muscle contraction to protein synthesis. The continuous production of ATP ensures that cells can perform their functions without interruption, enabling organisms to survive and thrive. The process of energy generation is tightly regulated to meet the needs of the cell without waste.
Through these processes, cells efficiently convert nutrients into usable energy, ensuring that the organism remains active and capable of responding to environmental changes. Without this continuous supply of energy, life would be unable to sustain itself.
ATP and Its Role in Metabolism
ATP serves as the primary energy source that powers most biological processes within cells. It provides the energy necessary for the various reactions involved in metabolism, which are crucial for maintaining cellular functions and sustaining overall organismal activity. Without ATP, many vital processes, such as growth, repair, and energy production, would cease to function.
Energy Transfer and Usage

The structure of ATP makes it highly effective for energy transfer. It consists of adenine, ribose, and three phosphate groups, which are linked by high-energy bonds. When one of these bonds is broken, energy is released and becomes available for use in biochemical reactions. This energy is critical for processes such as:
- Muscle Contraction: ATP is required for the contraction of muscle fibers, enabling movement.
- Protein Synthesis: ATP provides the necessary energy to link amino acids into proteins.
- Cell Division: Energy is needed to facilitate the processes of mitosis and meiosis.
Regeneration of ATP
Since ATP is continually consumed in cellular activities, it must be constantly regenerated. The cell uses processes like cellular respiration to convert nutrients into ATP. In aerobic conditions, oxygen is used to generate ATP efficiently, while in anaerobic conditions, less energy is produced but still enables vital functions.
The cycle of ATP generation and utilization ensures that cells can function without interruption, enabling organisms to carry out essential metabolic processes and adapt to ever-changing environments.
Structural Diversity of Biological Molecules
The remarkable variety in the structure of biological compounds allows them to perform a wide array of functions in living systems. These substances come in different shapes and sizes, which influence their role in processes such as energy storage, information transfer, and cellular communication. The complexity and adaptability of their structures make them integral to the proper functioning of organisms.
Different types of compounds can have vastly different structural features. Some are composed of simple building blocks, while others consist of complex networks of linked units. The diversity in the way these units are arranged and interact allows each compound to fulfill its specific role, whether in supporting the structure of cells, catalyzing reactions, or carrying genetic information.
This structural flexibility is essential for the dynamic nature of living systems, where compounds must constantly adapt to changing conditions and facilitate numerous biochemical reactions. Understanding the relationship between structure and function is key to appreciating how organisms maintain homeostasis and respond to environmental stimuli.
Hydrogen Bonds and Their Importance

Hydrogen bonds play a crucial role in maintaining the structure and function of various biological components. These interactions, though relatively weak individually, have a significant cumulative effect in stabilizing the shapes and behaviors of many essential substances in living organisms. From the structure of water to the function of DNA, hydrogen bonds are key to many vital processes.
Properties of Hydrogen Bonds
Hydrogen bonds form when a hydrogen atom, covalently bonded to an electronegative atom like oxygen or nitrogen, is attracted to another electronegative atom. This weak attraction can occur between molecules or within different parts of a large molecule. Despite their relative weakness, these bonds are highly effective in influencing the behavior of substances, especially in aqueous environments.
Biological Significance
Hydrogen bonds are integral to many processes in living systems:
- Water’s Properties: These bonds are responsible for water’s unique properties, such as its high surface tension and ability to dissolve a wide range of substances.
- Protein Folding: The three-dimensional shapes of proteins are largely stabilized by hydrogen bonds, which determine their function.
- DNA Structure: The double helix structure of DNA is held together by hydrogen bonds between complementary bases, ensuring the stability and accurate transmission of genetic information.
These interactions enable the formation of stable yet flexible structures, allowing organisms to maintain functionality and adapt to changes in their environment. The importance of hydrogen bonds cannot be overstated, as they are foundational to the chemistry of life itself.
The Role of Vitamins in Metabolism
Vitamins are essential nutrients that play key roles in regulating various metabolic pathways within the body. Though required in small amounts, they are critical for ensuring that the biochemical reactions responsible for energy production, growth, and repair function properly. Without these nutrients, many important processes would be hindered, leading to various health issues.
Vitamins as Coenzymes
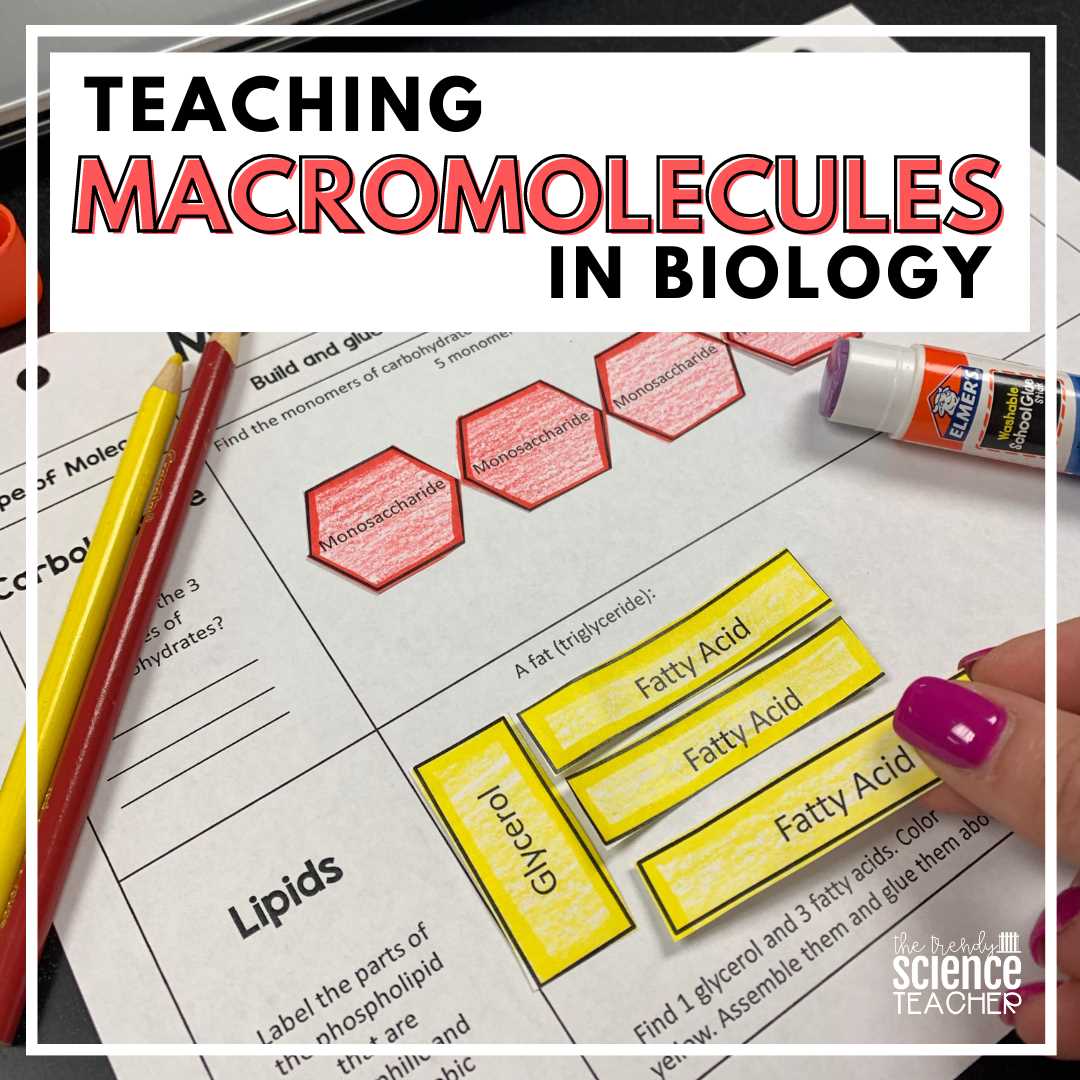
Many vitamins act as coenzymes or precursors to coenzymes, which are necessary for the proper functioning of enzymes involved in metabolic reactions. These coenzymes help accelerate biochemical processes by enabling enzymes to bind to substrates more effectively. Some vitamins, such as B-vitamins, are directly involved in energy production and the breakdown of nutrients.
- Vitamin B1 (Thiamine): Involved in carbohydrate metabolism and the production of energy.
- Vitamin B2 (Riboflavin): Plays a key role in cellular energy production through the metabolism of fats, proteins, and carbohydrates.
- Vitamin B3 (Niacin): Essential for the conversion of food into energy and supporting the function of the nervous system.
Supporting Metabolic Health
Vitamins also help maintain the balance of metabolic processes that regulate growth, repair, and immune function. They support antioxidant defense systems, prevent oxidative stress, and help with tissue healing. Deficiencies in certain vitamins can disrupt these processes, leading to conditions such as scurvy, rickets, or fatigue.
- Vitamin C: A powerful antioxidant that aids in collagen formation and protects cells from damage.
- Vitamin D: Important for bone health by supporting calcium absorption and regulating immune function.
- Vitamin A: Supports vision, skin health, and immune responses.
In summary, vitamins are integral to metabolism, ensuring the efficient conversion of nutrients into usable energy and supporting overall health. Their varied functions highlight their importance in maintaining the body’s complex biochemical systems.
The Importance of Molecular Interactions
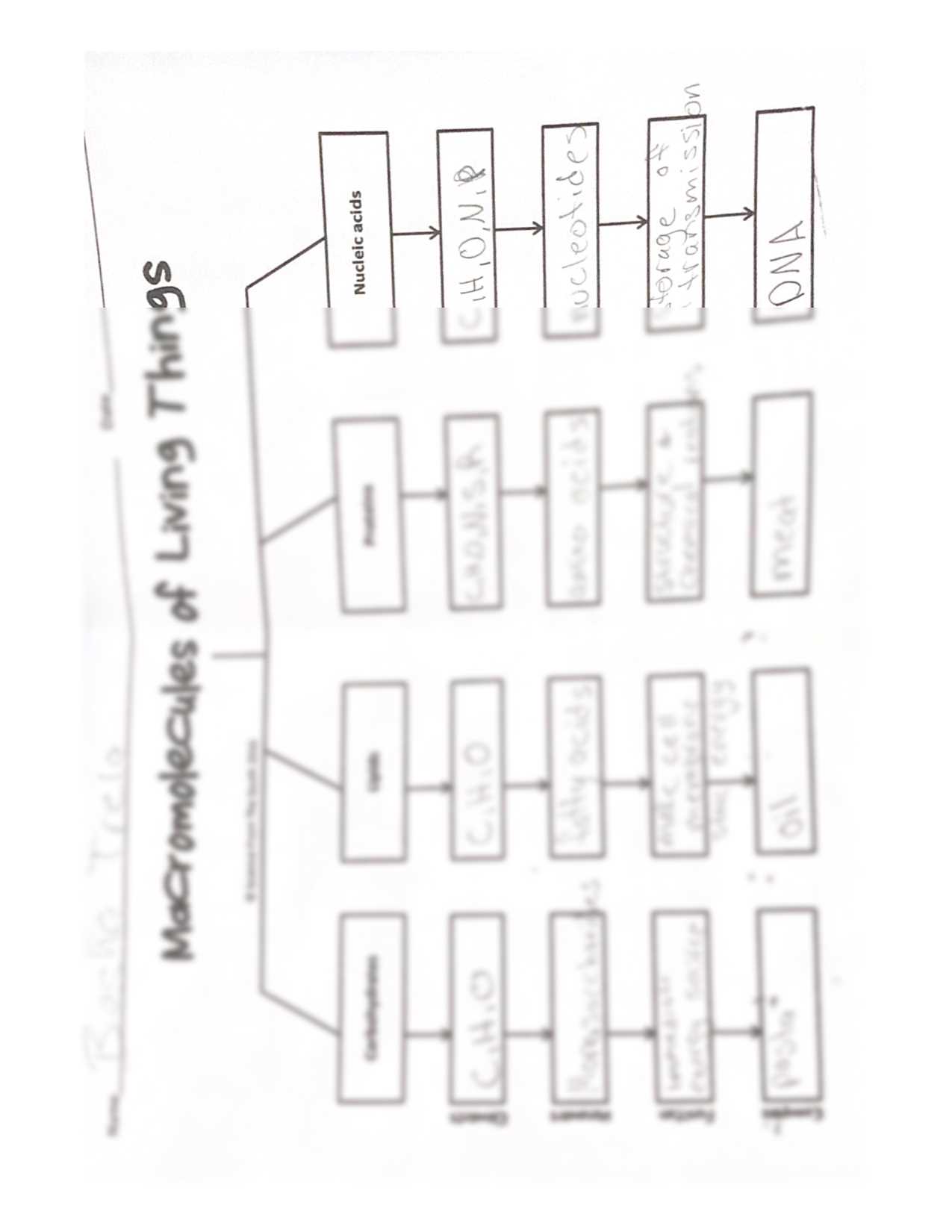
The interactions between different types of chemical entities are fundamental to the functioning of living systems. These interactions dictate how substances combine, form structures, and carry out specific tasks within cells. Without these precise associations, processes such as energy transfer, signaling, and the synthesis of complex compounds would not be possible.
Types of Interactions
Various types of chemical interactions contribute to the stability and functionality of biological systems. These interactions are generally classified based on the strength and nature of the bonds formed:
- Covalent Bonds: Strong bonds where electrons are shared between atoms, forming stable structures such as proteins and nucleic acids.
- Hydrogen Bonds: Weaker bonds that play a crucial role in maintaining the structure of DNA and proteins, as well as in the properties of water.
- Van der Waals Forces: Temporary, weak attractions that help molecules interact at short distances and are important in the folding of proteins.
- Ionic Bonds: Formed when atoms with opposite charges attract each other, contributing to the structure of salts and the function of enzymes.
Biological Functions Driven by Interactions

The complex network of interactions in living systems ensures that every biological function is carried out with precision:
- Enzyme Activity: Specific binding between enzymes and their substrates accelerates chemical reactions vital for metabolism.
- Cell Communication: Signaling molecules interact with receptors on cell surfaces to trigger responses that regulate processes like growth and immune defense.
- DNA Replication: The complementary base pairing in DNA ensures accurate copying of genetic material during cell division.
In essence, the myriad of interactions that occur within cells enable the smooth operation of biological processes, maintaining the balance required for health and function. Understanding how these interactions work provides insight into the underlying principles that govern cellular life.
Exploring the Chemistry of Life
The chemistry within living organisms is a complex web of interactions that enable essential functions such as growth, energy production, and cellular communication. This intricate network of chemical reactions and structures forms the foundation of all biological activities. Understanding how these reactions work is key to comprehending how life maintains itself and adapts to its environment.
The Building Blocks of Biological Systems
At the heart of every living system are the basic building blocks that drive chemical reactions. These compounds interact with one another to form larger, more complex structures. Key categories include:
- Proteins: Long chains of amino acids that fold into specific shapes, enabling them to catalyze reactions, transport substances, and perform structural functions.
- Carbohydrates: Sugars and starches that provide energy and serve as structural components in cells.
- Lipids: Fatty substances that form the structural foundation of cell membranes and store energy.
- Nucleic Acids: DNA and RNA, which store and transmit genetic information necessary for growth and reproduction.
The Role of Chemical Reactions in Metabolism
Biological processes are powered by chemical reactions, which occur in tightly regulated pathways. These reactions allow organisms to convert nutrients into energy, synthesize complex molecules, and maintain cellular structures. Enzymes, proteins that catalyze these reactions, lower the activation energy needed for reactions to take place, ensuring that metabolic processes occur efficiently and at the right time.
The study of the chemistry within organisms not only helps us understand how life operates on a cellular level but also provides insights into disease processes, the development of new therapies, and the enhancement of biotechnological advancements. It is through this lens that we continue to explore the fundamental principles that make life possible.