Nuclear Chemistry Study Guide Answers

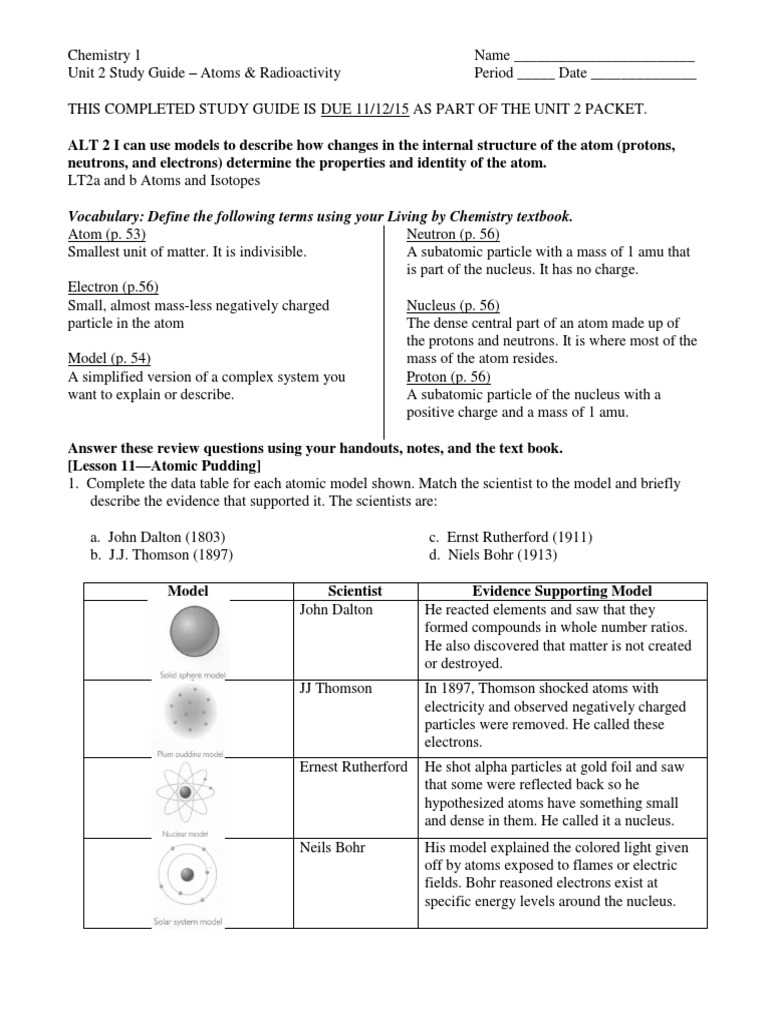
The study of atomic interactions and energy transformations plays a crucial role in various scientific and industrial applications. Understanding how atoms behave under certain conditions can lead to significant advancements in technology, medicine, and energy production. This field offers insights into fundamental processes that shape the natural world, from radiation to energy release.
By exploring the principles behind atomic structure and its changes, learners can gain a deeper appreciation for the complexities of matter at a microscopic level. These concepts are essential for anyone looking to grasp how powerful forces work within the atom and how they are harnessed for practical use. The key to mastering this subject lies in breaking down these concepts into manageable parts and recognizing their real-world implications.
Whether you are preparing for exams or seeking to enhance your understanding, focusing on the foundational principles and their applications will provide a solid base for further exploration. With the right approach, anyone can grasp these topics and use them to further their scientific knowledge and practical skills.
Understanding the Basics of Radioactive Processes
The fundamental principles behind atomic reactions and energy changes are essential for grasping the core of this field. Atoms are constantly in motion, and under certain conditions, they can undergo transformations that release or absorb energy. These processes, which are pivotal in numerous applications, allow us to understand the behavior of matter at its most fundamental level. Recognizing how atoms react with each other and the forces involved is key to understanding how these changes take place and how they can be controlled or harnessed for practical use.
To begin, it is important to familiarize yourself with the building blocks of atoms and how they interact. The nucleus, made up of protons and neutrons, is at the heart of most reactions, with electrons surrounding it. Understanding how these subatomic particles influence the overall behavior of an atom will provide insight into the larger processes that take place during various reactions.
| Term | Description |
|---|---|
| Proton | A positively charged particle located in the nucleus of an atom. |
| Neutron | A neutral particle found in the nucleus alongside protons. |
| Electron | A negatively charged particle that orbits the nucleus of an atom. |
| Isotope | Atoms of the same element with a different number of neutrons. |
Through a deeper exploration of atomic structure and the forces at play, you can begin to see how atoms can change and release energy. Whether it’s through spontaneous disintegration or by interaction with other elements, understanding these processes is the first step in mastering the broader subject and its practical applications. The journey of learning starts with grasping these essential principles and their significance in real-world contexts.
Key Concepts in Atomic Reactions
Understanding the underlying principles of atomic transformations is crucial for grasping the full scope of energy release and absorption. These reactions, which involve changes within the atom’s core, can result in significant alterations to the matter itself. By examining how atoms interact, break apart, or combine, we uncover the mechanisms driving energy production and elemental change. Whether these processes are harnessed for practical uses or occur naturally, understanding them allows us to better control and predict the outcomes.
One of the primary concepts in atomic reactions is the concept of stability. Atoms, depending on their structure and the forces acting within, may undergo spontaneous changes to achieve a more stable state. This transformation can release energy in the form of radiation or heat, which is often used in various fields, such as energy production and medical treatments. Additionally, understanding the difference between various types of reactions, such as fission and fusion, helps clarify how energy is harnessed or released in controlled environments.
Another important concept is the role of isotopes. Atoms of the same element can have different numbers of neutrons, which makes them more or less stable. The balance between protons and neutrons plays a vital role in determining the behavior of the atom during reactions, influencing how energy is produced or how the atom interacts with other elements.
Types of Radiation in Atomic Reactions
Radiation is a form of energy emitted by unstable atomic nuclei as they transform into more stable configurations. These emissions can take different forms, each with distinct properties and behaviors. Understanding the types of radiation is crucial for recognizing how energy is released and how it interacts with matter. The nature of these emissions determines their potential uses, as well as their hazards in certain environments.
There are three primary types of radiation that occur during atomic transformations: alpha, beta, and gamma radiation. Each type has a unique impact on the material it interacts with, varying in penetration power and potential to cause damage. By studying the characteristics of each radiation type, we can better understand how to protect against harmful effects while taking advantage of their beneficial uses.
| Type of Radiation | Properties | Penetration Power | Common Sources |
|---|---|---|---|
| Alpha Radiation | Consists of two protons and two neutrons | Low; can be stopped by paper or skin | Uranium, Radon |
| Beta Radiation | Consists of high-energy electrons | Moderate; can penetrate paper but is stopped by plastic or glass | Carbon-14, Strontium-90 |
| Gamma Radiation | Electromagnetic waves (high-energy photons) | High; requires thick lead or concrete shielding | Cobalt-60, Cesium-137 |
Each type of radiation is associated with specific applications, such as medical treatments, industrial processes, and energy generation. However, the potential risks also demand careful handling and protection. Understanding these different types of emissions helps ensure that their benefits are harnessed safely and efficiently while minimizing their harmful effects on both humans and the environment.
How Atomic Decay Affects Elements
When an unstable atomic nucleus undergoes transformation, it releases energy and particles, which leads to the alteration of the original atom. This process, known as decay, results in the formation of a different element or isotope. Over time, these transformations can dramatically change the composition of matter, influencing both the properties of substances and their behavior in natural and technological contexts. Understanding how this process works is essential for predicting the stability and reactions of various elements.
During decay, the nucleus of an atom may emit particles such as alpha particles, beta particles, or gamma rays, depending on the type of decay occurring. These emissions can cause the atom to lose or gain protons, changing its atomic number and thus transforming it into a new element. For example, when uranium decays, it eventually forms lead, passing through several intermediate steps along the way. This process can take millions or even billions of years, but its impact on the atom is immediate, altering its chemical and physical properties.
The impact of decay extends beyond just the element being transformed. As atoms release energy and particles, they can interact with surrounding matter, causing further reactions and changes. This is particularly important in fields such as medicine, where controlled decay is used in treatments like radiation therapy, and in energy production, where it is harnessed to generate electricity. The study of how atomic decay affects elements provides crucial insights into both natural processes and human applications.
Radioactive Isotopes and Their Uses
Isotopes with unstable nuclei, commonly known for their ability to emit radiation, have found a wide range of applications in various fields. These isotopes, often referred to as radioactive, differ from stable ones by their excess energy or mass, making them prone to decay. While they can be hazardous in some contexts, their unique properties also make them incredibly useful in medicine, industry, and research.
Applications in Medicine
In the medical field, radioactive isotopes are used to diagnose and treat various conditions. These isotopes can help visualize internal organs, detect diseases, and target tumors. Some of the common applications include:
- Diagnostic Imaging: Radioactive tracers are injected into the body to provide clear images of organs and tissues. For example, iodine-131 is commonly used for thyroid imaging.
- Cancer Treatment: Certain isotopes, like cobalt-60, are used in radiation therapy to target and destroy cancer cells while minimizing damage to healthy tissue.
- Bone Scans: Technetium-99m is widely used to scan bones and detect abnormalities such as fractures or infections.
Industrial and Research Uses
In addition to medicine, radioactive isotopes have significant industrial and research applications:
- Radiography: Isotopes like iridium-192 are used in non-destructive testing to inspect the integrity of materials and structures such as pipelines and metal welds.
- Radiation Sterilization: Cobalt-60 is used to sterilize medical equipment, food, and packaging by killing bacteria and pathogens.
- Carbon Dating: Carbon-14 is essential in archaeology and geology for determining the age of ancient artifacts and fossils by measuring the decay of carbon isotopes.
These applications highlight how radioactive isotopes contribute to advancements in science, technology, and healthcare, despite their inherent risks. By harnessing their unique properties in controlled environments, society can benefit from their wide range of uses while ensuring safety through proper handling and precautions.
Understanding Half-Life in Atomic Reactions
In many atomic transformations, the time it takes for half of a given sample to undergo change is an important concept. This time period, known as half-life, is a measure of how long it takes for half of the atoms in a substance to decay or change into another form. The concept of half-life is fundamental in understanding the behavior of unstable atoms and plays a critical role in various fields, from dating ancient materials to controlling radiation in medical treatments.
How Half-Life Works
During a transformation process, atoms decay at a predictable rate, and the half-life is the amount of time it takes for half of the atoms in a sample to decay. This means that after one half-life, 50% of the original substance will have transformed, and after two half-lives, only 25% of the original material will remain. This process continues until the material becomes stable or its decay products are no longer detectable.
- Consistency: The half-life is constant for a given substance, regardless of the amount of material present. This predictable nature makes it useful for various applications.
- Exponential Decay: The decay of atoms follows an exponential pattern, meaning the rate of decay decreases over time as fewer atoms remain to undergo transformation.
- Time Measurement: The half-life is a critical time marker, used to estimate the age of objects or determine how long a material will remain radioactive.
Applications of Half-Life
Understanding half-life is crucial in several fields, especially in archaeology, medicine, and energy production:
- Radiocarbon Dating: By measuring the amount of carbon-14 left in ancient artifacts or fossils, scientists can estimate their age, thanks to the predictable half-life of this isotope.
- Medical Treatments: Half-life is important in managing the dosage of radioactive materials used in cancer therapy, ensuring that patients receive the correct amount of treatment over time.
- Environmental Monitoring: In managing radioactive waste or assessing contamination levels, understanding half-life helps determine how long it will take for materials to lose their dangerous properties.
Overall, the concept of half-life helps make sense of how unstable materials behave over time. It provides a reliable method for calculating decay patterns and offers essential insight into the lifespan of various substances in both natural and controlled environments.
Calculating Atomic Fission Reactions

Atomic fission involves the splitting of a large, unstable nucleus into smaller nuclei, releasing a significant amount of energy in the process. This reaction is the foundation of various energy-producing technologies, such as nuclear power plants. Calculating the energy released and the products formed during fission is essential for understanding how these reactions work and how they can be controlled. The process involves several key steps, such as determining the mass loss and applying energy conversion formulas to estimate the released energy.
Steps in Calculating Fission Energy
To calculate the energy produced during a fission reaction, the mass defect (the difference between the mass of the original nucleus and the mass of the fission products) must be determined. This mass defect is then converted into energy using Einstein’s famous equation, E=mc². Here’s an overview of the steps involved in the calculation:
- Identify the initial nucleus: Determine the mass of the atom before fission occurs, typically uranium-235 or plutonium-239.
- Determine the fission products: The splitting of the nucleus results in smaller nuclei, often along with neutrons and energy. Find the masses of these products.
- Calculate the mass defect: Subtract the combined mass of the fission products from the original mass of the nucleus.
- Convert mass defect to energy: Use the equation E=mc² to calculate the energy released in the reaction.
Example of Fission Reaction Calculation
Let’s consider the fission of a uranium-235 atom. When it undergoes fission, it typically splits into two smaller nuclei, along with the release of additional neutrons and energy. To calculate the energy released:
- Step 1: Find the mass of the uranium-235 nucleus and the resulting fission products (e.g., barium-141, krypton-92, and additional neutrons).
- Step 2: Calculate the mass defect (the difference in mass before and after fission).
- Step 3: Use the equation E=mc² to convert the mass defect into energy, which is typically measured in mega-electron volts (MeV).
By following these steps, it is possible to estimate the amount of energy produced by a fission reaction, which is crucial for understanding how much energy can be harnessed for power generation or other applications. Calculating these reactions also plays an important role in ensuring the safety and efficiency of nuclear processes.
Exploring the Process of Fusion
Fusion is the process where two light atomic nuclei combine to form a heavier nucleus, releasing a tremendous amount of energy in the process. This reaction powers the sun and stars, and scientists have been attempting to replicate this energy production on Earth. Unlike splitting large atoms, fusion involves merging smaller atoms under extreme conditions of temperature and pressure, which makes it both a highly efficient and challenging method of energy generation.
The Steps Involved in Fusion
For fusion to occur, the particles involved must overcome the repulsive forces between them due to their positive charges. This requires extremely high temperatures and pressures, which is why fusion reactions are most commonly observed in stars or experimental reactors. The main steps in the process are:
- High temperature and pressure: The nuclei must be heated to millions of degrees Celsius, creating conditions where particles move fast enough to collide with each other and overcome electrostatic repulsion.
- Particle collision: Under these extreme conditions, the light nuclei, such as hydrogen isotopes, collide and fuse together, forming a heavier element, like helium.
- Energy release: The mass of the new nucleus is slightly less than the sum of the original particles. This “missing” mass is converted into energy, according to Einstein’s equation, E=mc².
Fusion in Practical Applications
While fusion has the potential to provide a nearly limitless and clean source of energy, replicating the process on Earth is incredibly difficult. However, there are several areas where fusion research is focused:
- Energy Production: The primary goal of fusion research is to develop reactors that can safely and efficiently produce energy. Projects like ITER (International Thermonuclear Experimental Reactor) aim to make this a reality.
- Space Exploration: Fusion reactions could potentially provide the energy needed for long-duration space missions, as they produce more energy per unit of fuel than conventional chemical reactions.
- Hydrogen Fuel: By fusing isotopes of hydrogen, fusion offers a cleaner alternative to fossil fuels, as the primary byproduct of the reaction is non-radioactive helium.
Though challenges remain in achieving practical and controlled fusion, progress continues, and the potential benefits of mastering fusion reactions could revolutionize energy production for generations to come.
The Role of Neutrons in Atomic Reactions
Neutrons play a crucial role in the transformation of atomic nuclei, influencing the stability and behavior of elements. These neutral particles, which do not carry an electric charge, are essential in various reactions, including the initiation of processes like fission and fusion. Their ability to penetrate the nuclei of atoms without being repelled by the positive charge makes them valuable tools for both scientific research and energy generation.
How Neutrons Interact with Atoms
In many reactions, neutrons act as initiators or catalysts, enabling the splitting of larger nuclei or the fusion of smaller ones. When a neutron collides with a nucleus, the nucleus may absorb the neutron and become unstable, leading to a variety of reactions. This process is fundamental in the creation of energy in reactors, as well as in the formation of new elements through nuclear reactions.
- Neutron absorption: When an atom absorbs a neutron, it may become unstable and decay, or undergo fission, releasing more neutrons and energy.
- Neutron scattering: Neutrons can also scatter off atoms, altering their energy levels or causing other atomic rearrangements.
- Neutron moderation: In reactors, neutrons are slowed down (moderated) to increase the probability of inducing fission in fuel atoms, such as uranium or plutonium.
Applications of Neutrons
The use of neutrons extends far beyond energy production. Their ability to interact with matter in unique ways has made them valuable in several fields:
- Medical treatments: Neutron therapy is used to treat certain cancers by targeting tumor cells with neutron radiation.
- Scientific research: Neutrons are used in particle accelerators and reactors for research in physics, material science, and biology. Neutron scattering, for example, is a technique used to analyze the structure of materials at the atomic level.
- Energy production: Neutrons are key in the operation of reactors, where their interactions with fuel elements sustain the chain reactions necessary for power generation.
In conclusion, neutrons are indispensable in both natural processes and engineered systems. Their ability to initiate reactions, facilitate energy release, and support scientific discovery makes them one of the most important particles in atomic science.
Applications of Radiation Science in Medicine
The principles of radiation science have found numerous applications in medicine, offering powerful tools for diagnosing and treating various health conditions. By harnessing the energy released from atomic interactions, medical professionals can gain detailed insights into the human body, monitor disease progression, and even treat certain types of cancer. These technologies have revolutionized modern healthcare, making them indispensable in both clinical and research settings.
Diagnostic Techniques
One of the most important uses of radiation in healthcare is in imaging techniques, which allow doctors to view the internal structures of the body without the need for invasive surgery. These methods utilize different forms of radiation to create detailed images, helping to diagnose a wide range of conditions:
- X-rays: X-ray imaging is widely used to examine bones, detect fractures, and identify abnormalities such as tumors or infections.
- Positron Emission Tomography (PET): PET scans provide detailed images of metabolic processes, helping to diagnose cancer, heart disease, and brain disorders.
- Single-Photon Emission Computed Tomography (SPECT): Similar to PET, SPECT scans are used to monitor blood flow and assess organ function, particularly in the brain and heart.
- CT Scans: Computed tomography uses multiple X-ray images combined with computer processing to create cross-sectional views of the body, aiding in the detection of various medical conditions.
Therapeutic Uses
Radiation therapy is another key application of radiation in medicine. This treatment uses high-energy radiation to target and destroy cancer cells, often shrinking tumors or halting their growth. In addition to cancer treatment, radiation is also employed in:
- Radioactive Iodine Therapy: This method is used to treat thyroid cancer or hyperthyroidism by administering radioactive iodine, which selectively targets thyroid cells.
- Brachytherapy: This involves placing radioactive sources directly inside or near a tumor, providing high-dose radiation to cancer cells while minimizing damage to surrounding healthy tissue.
- Bone Pain Palliation: Radiotherapy is used to relieve pain from metastatic cancer in bones, improving the quality of life for patients with advanced-stage cancer.
In conclusion, the application of radiation in the medical field continues to expand, offering new opportunities for diagnosis, treatment, and patient care. Its ability to provide precise and targeted therapies, as well as its role in improving diagnostic accuracy, ensures that radiation science will remain a cornerstone of modern medicine for the foreseeable future.
Safety Precautions in Radiation Laboratories
Working with radioactive materials and high-energy radiation requires strict adherence to safety protocols to protect both personnel and the environment. Laboratories handling such substances must implement rigorous safety measures to prevent exposure, contamination, and accidents. These protocols are designed to minimize risks and ensure that all procedures are conducted in a controlled and secure manner.
Personal Protective Equipment (PPE)
To safeguard individuals working in radiation labs, personal protective equipment is essential. PPE helps to limit direct contact with hazardous substances and provides a barrier against potential radiation exposure. Commonly used protective gear includes:
- Lead aprons: These are worn to protect the body from harmful radiation, particularly when working with X-rays or gamma rays.
- Gloves: Specialized gloves are used to prevent contamination when handling radioactive materials or equipment.
- Eye protection: Safety goggles or face shields are worn to shield the eyes from radiation and potential chemical splashes.
- Respirators: In some cases, respirators are used to prevent inhaling radioactive particles or fumes.
Proper Storage and Handling
Safe storage and handling practices are critical in preventing accidental exposure to radiation. Radioactive materials should be securely stored in shielded containers that limit radiation leakage. Laboratories also follow specific procedures when handling materials to ensure minimal risk of contamination:
- Sealed containers: Radioactive substances are kept in tightly sealed containers to prevent accidental release into the environment.
- Labeling: All radioactive materials are clearly labeled with hazard warnings, ensuring that workers are aware of the potential risks.
- Shielding: Work areas are equipped with shielding such as lead or concrete to absorb or deflect harmful radiation.
In addition to personal protective measures and safe handling, laboratories must regularly monitor radiation levels to ensure they remain within safe limits. Regular inspections, proper disposal of waste, and adherence to regulatory guidelines are all part of maintaining a safe working environment. By following these precautions, radiation laboratories can operate securely, minimizing health risks to workers and preventing accidents that could affect public safety.
Environmental Impact of Radiation Science
The use of radioactive materials in various industries and research has significant implications for the environment. While the advancements in science and technology have provided benefits such as clean energy and medical treatments, they have also led to concerns about the long-term effects of radiation exposure on ecosystems. Managing these impacts requires careful monitoring and adherence to safety standards to prevent contamination and ensure the sustainable use of radioactive substances.
Key Areas of Environmental Impact
There are several critical areas where the use of radiation can affect the environment:
- Contamination of Water Sources: Accidental spills or improper disposal of radioactive waste can lead to contamination of water sources, which may affect aquatic life and the communities relying on those water supplies.
- Soil Pollution: Radioactive materials that are improperly stored or disposed of can leach into the soil, affecting the fertility of land and potentially entering the food chain through plants and animals.
- Air Quality: Emissions from nuclear power plants, research facilities, or during mining operations can release radioactive particles into the air, leading to potential health risks for nearby populations and wildlife.
Efforts to Minimize Environmental Impact
Efforts to mitigate the environmental impact of radiation include regulatory measures, advancements in technology, and improved waste management practices. Some key approaches include:
| Strategy | Description |
|---|---|
| Regulatory Controls | Governments and organizations set strict guidelines and regulations for the handling, disposal, and storage of radioactive materials to reduce environmental risks. |
| Waste Management | Advanced technologies are used to safely store or neutralize radioactive waste, ensuring it does not leak into the environment. |
| Environmental Monitoring | Continuous monitoring of radiation levels in air, water, and soil helps detect contamination early and minimize its spread. |
| Public Awareness | Educating the public about radiation safety and environmental protection helps reduce exposure risks and encourages responsible use of radioactive materials. |
While there are unavoidable risks associated with radiation use, advances in safety measures, waste management, and technology continue to reduce the environmental impact. A balanced approach is necessary to ensure that the benefits of radiation applications outweigh the potential harms to the environment.
Principles of Energy Production through Atomic Reactions
Energy production through atomic processes relies on harnessing the immense power stored within the core of atoms. This process involves the manipulation of atomic structures to release energy, either through splitting large atoms or combining smaller ones. These methods are pivotal in providing a substantial portion of the world’s energy needs, particularly in power plants that generate electricity for large populations. Understanding the fundamental mechanisms that drive these processes is essential for their safe and efficient implementation.
Key Mechanisms of Energy Generation
The process of generating energy from atomic reactions typically involves either fission or fusion. These two methods differ in their approach but share the goal of producing energy through the alteration of atomic nuclei:
- Fission: In this process, a heavy atom is split into smaller nuclei, releasing a significant amount of energy. This reaction is typically initiated by bombarding the atom with a neutron. The released energy is captured and converted into electricity.
- Fusion: Fusion involves combining two light atomic nuclei to form a heavier nucleus, releasing energy in the process. This reaction occurs naturally in stars, including the Sun, but achieving controlled fusion on Earth is an ongoing area of research.
Energy Conversion and Efficiency
Once the energy is released from atomic reactions, it must be converted into a usable form, typically electricity. The conversion process in power plants typically follows these steps:
- Heat Generation: The fission or fusion reaction generates immense heat, which is used to heat water and produce steam.
- Turbine Movement: The steam generated in the previous step is directed onto turbines, causing them to spin. This mechanical movement is the key to driving generators.
- Electricity Generation: The spinning turbines are connected to electrical generators, which convert mechanical energy into electrical energy for distribution.
The efficiency of energy production depends on several factors, including the type of reaction used, the materials involved, and the technology available to capture and convert the released energy. Despite its potential, energy generation through atomic reactions requires careful management to ensure safety, minimize waste, and optimize performance.
Understanding Atomic Science in Industry
The use of atomic processes in various industries has revolutionized numerous fields, from energy production to medicine. These techniques involve the manipulation of atomic particles to achieve specific results, such as energy generation, material enhancement, and medical diagnostics. Industries across the globe rely on this science to improve efficiency, safety, and overall productivity. Understanding the underlying principles of atomic science is key to advancing these applications and ensuring their sustainable and safe use.
Applications in Energy Production
One of the most significant applications of atomic science in industry is energy production. Power plants harness atomic reactions to generate electricity, supplying vast amounts of power to cities and industries. By using controlled atomic reactions, these plants release energy that is converted into heat, which drives turbines connected to electricity generators. The most common reaction used for energy generation is the splitting of heavy atoms, which produces a large amount of energy.
Use in Medicine and Diagnostics
Atomic science plays a crucial role in the field of medicine, particularly in diagnostic imaging and cancer treatment. Radioactive isotopes are employed in various diagnostic procedures, such as PET scans and X-rays, to observe internal body structures and monitor disease progression. Additionally, atomic techniques are used in targeted radiation therapy to treat specific areas affected by cancer, allowing for more precise and effective treatments while minimizing damage to surrounding healthy tissues.
In addition to these applications, the manipulation of atomic particles is used in industries such as materials science, where it helps develop stronger and more durable materials, and in environmental monitoring, where it assists in detecting pollutants and ensuring safety standards. With continued advancements, the potential for atomic science to improve various industrial processes remains vast.
Common Mistakes in Atomic Science Studies
Learning the principles of atomic science can be complex, and students or professionals in the field often encounter challenges. Some common errors arise from misunderstandings of key concepts, improper calculations, or overlooking safety measures. These mistakes can hinder progress, lead to inaccurate conclusions, and even result in hazardous situations. Identifying and addressing these common pitfalls is crucial to mastering the subject and ensuring success in real-world applications.
Misunderstanding of Atomic Reactions

A frequent mistake involves misinterpreting atomic reactions and their outcomes. For example, students might confuse the processes of fission and fusion, assuming that they are similar when, in fact, they are fundamentally different. In fusion, atoms combine to release energy, whereas in fission, atoms split. Incorrectly equating these two processes can lead to significant misconceptions about how energy is generated in atomic reactions. Understanding the distinction between these reactions is essential for both theoretical and practical applications in the field.
Incorrect Calculation of Half-Life
Another common mistake involves the calculation of half-life, which is crucial when dealing with radioactive materials. Many people mistakenly assume that half-life is the same for all substances or neglect to account for variations in decay rates between different isotopes. For accurate results, it’s vital to use the correct formula and data specific to each isotope. Failing to do so can lead to errors in predictions related to radiation levels, contamination, and safety procedures.
Inaccurate data analysis is also a major issue, as it can result from improper measurement techniques or failure to interpret experimental results correctly. Regular review of fundamental principles and consistent practice with calculations and problem-solving can help mitigate these errors.
By recognizing and addressing these common mistakes, students and professionals can improve their understanding of atomic science and apply their knowledge more effectively in both research and industrial contexts.
Study Tips for Mastering Atomic Science
Mastering atomic science requires not only theoretical understanding but also practical application and problem-solving skills. Whether you’re a student starting your journey or a professional looking to deepen your knowledge, adopting effective study techniques can make all the difference. These tips are designed to help you engage with complex concepts, retain information, and apply your knowledge confidently in real-world scenarios.
1. Focus on Core Concepts
The foundation of atomic science lies in understanding core principles, such as atomic structure, radiation types, and energy production. Begin by breaking down the subject into manageable parts and focus on mastering one concept before moving to the next. Build your understanding step by step, as attempting to tackle advanced topics without grasping the basics can lead to confusion.
2. Use Visual Aids and Models
Visual aids, such as diagrams, charts, and models, can help clarify complex processes. Atomic reactions, for example, can be difficult to visualize without a clear representation. Using models to understand atomic behavior, decay processes, and particle interactions can significantly enhance your comprehension. Additionally, interactive simulations and online resources provide dynamic ways to explore concepts and reinforce your learning.
3. Practice Problem-Solving
Atomic science involves a great deal of calculation and problem-solving, particularly when it comes to reaction rates, decay, and energy conversion. Regularly practicing problems will not only solidify your understanding but also improve your ability to apply theoretical concepts to practical situations. Start with simple problems and gradually progress to more complex scenarios. Working through problems helps you identify common mistakes and learn how to avoid them.
4. Collaborate and Discuss
Collaborating with peers or joining study groups can be extremely beneficial. Discussing difficult concepts with others can help clarify your understanding, provide new perspectives, and expose you to different problem-solving methods. Don’t hesitate to ask questions or explain concepts to others–teaching is often the best way to reinforce your own knowledge.
5. Stay Organized and Consistent
Consistency is key to mastering any subject. Create a study schedule that allows you to review material regularly and track your progress. Use a combination of reading, note-taking, and active recall techniques to reinforce your understanding. Staying organized with your notes, assignments, and resources will also help you quickly access the information you need when studying or preparing for exams.
By incorporating these strategies into your study routine, you can build a strong foundation in atomic science and confidently apply your knowledge in various contexts.