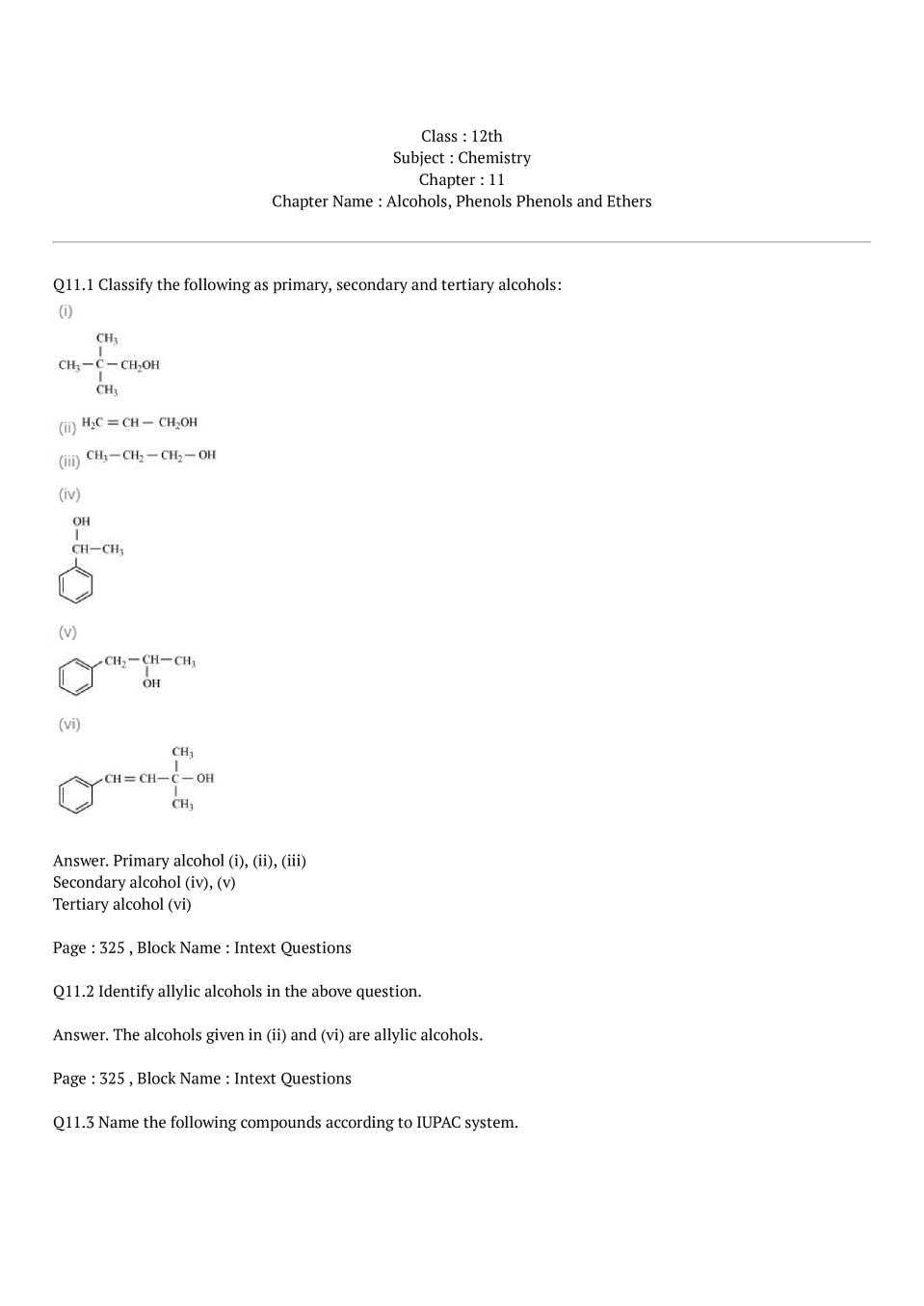
Chemistry Chapter 7 Test Answers

Understanding the fundamental principles of the natural world is essential for excelling in any academic field related to the physical sciences. Whether you are preparing for an exam or looking to strengthen your knowledge, grasping core ideas plays a crucial role in achieving success. The material covered in this section focuses on the important concepts that often appear in assessments and practical applications.
Key topics in this segment include the analysis of various reactions, the use of mathematical calculations to predict outcomes, and the identification of crucial patterns that govern the behavior of matter. By breaking down these complex topics into manageable pieces, students can build a stronger foundation for more advanced studies. Strategic learning through practice and problem-solving is the best way to solidify your understanding.
Understanding Key Concepts in Science

Grasping the core principles that govern the behavior of matter is essential for mastering this subject. These concepts form the foundation for solving problems and applying theoretical knowledge to real-world scenarios. To succeed, it’s crucial to understand how different elements and compounds interact, as well as the mathematical relationships that help predict their behavior.
One of the most important aspects of this subject is the ability to balance chemical reactions and understand the stoichiometry involved. These skills are critical for solving problems and explaining observed phenomena. Below is a table highlighting some of the key areas to focus on when building your understanding:
| Concept | Description | Importance |
|---|---|---|
| Reactions | Understanding how substances interact to form new compounds. | Critical for solving real-world scientific problems. |
| Stoichiometry | Calculation of reactants and products in chemical reactions. | Essential for determining the outcome of reactions accurately. |
| Balancing Equations | Ensuring that the number of atoms on both sides of a reaction is equal. | Vital for maintaining consistency and accuracy in chemical equations. |
| Periodic Trends | Understanding the patterns that elements follow across the periodic table. | Helps in predicting the behavior of elements in reactions. |
By focusing on these foundational topics, students can develop a deeper understanding and improve their ability to apply knowledge in practical situations. Mastery of these core ideas will also build confidence when tackling more complex problems.
Exploring Chemical Reactions in Chapter 7
Understanding how substances interact and transform during reactions is a fundamental part of studying science. These processes not only explain the changes that occur in various environments but also provide insight into the laws that govern matter. The reactions explored in this section showcase how elements combine or break apart to form new substances, often with distinct properties.
One of the most crucial aspects to focus on is identifying the different types of reactions. Each reaction type follows specific patterns, and recognizing these patterns is key to predicting the products of a given reaction. Whether it’s a combination, decomposition, or displacement reaction, knowing the underlying mechanisms helps simplify complex problems.
In addition to understanding the types of reactions, it is equally important to balance the reactions correctly. This ensures that the number of atoms remains constant on both sides of the equation, in line with the principle of conservation of mass. Proper balancing is essential for accurate calculations and predictions of the reaction’s outcome.
Common Mistakes to Avoid in Science Assessments
When preparing for scientific evaluations, it’s important to be aware of common pitfalls that can lead to errors in your answers. These mistakes often arise from misunderstandings of basic concepts or rushing through problems without careful consideration. Avoiding these errors can make a significant difference in your performance and help you better apply your knowledge.
One of the most frequent mistakes is neglecting to carefully read the question. Often, students skip over important details that can drastically affect the outcome. Paying attention to each part of the question is crucial for providing the correct response.
Another common issue is making errors in calculations, especially when it comes to conversions or balancing reactions. It’s essential to double-check your work and ensure that all units are consistent and that all steps are followed accurately. Skipping these verification steps can lead to avoidable mistakes, even if the initial approach was correct.
Breaking Down Chemical Equations
Understanding how to interpret and balance chemical reactions is essential for mastering the subject. Chemical equations represent the transformation of reactants into products, and breaking them down into their components helps clarify the process. By analyzing each part of an equation, you can gain insights into the amounts of substances involved and ensure that mass is conserved throughout the reaction.
To break down a chemical equation effectively, follow these steps:
- Identify the reactants and products: These are the substances that undergo change and the resulting substances formed after the reaction.
- Count the atoms: Ensure that the number of atoms for each element is the same on both sides of the equation, following the principle of conservation of mass.
- Balance the equation: Adjust the coefficients in front of the chemical formulas to make sure that the number of atoms on the left side matches the number on the right side.
By mastering these steps, you’ll be able to approach equations with confidence and avoid common mistakes. Remember, practice is key–solving different types of equations will help you recognize patterns and become more efficient at balancing reactions.
Tips for Effective Test Preparation
Preparing for an evaluation requires more than just reviewing material–it’s about developing a strategy that maximizes your retention and boosts your confidence. Effective preparation involves understanding key concepts, practicing regularly, and organizing your study time to ensure you cover all the necessary material.
Organize Your Study Sessions
Proper planning is crucial for effective revision. Break your study time into focused sessions, allowing enough time for each topic. Follow these steps to organize your preparation:
- Set specific goals: Identify what you need to master and create a clear outline of topics to cover.
- Use active recall: Test yourself on concepts rather than simply rereading notes. This reinforces memory retention.
- Space out your sessions: Avoid cramming by spreading study sessions over several days to improve long-term retention.
Practice Problem-Solving
Consistent practice is one of the best ways to improve your skills. Work through a variety of problems related to the topics being covered. Here are a few strategies:
- Practice with old materials: Solve previous assignments or mock exams to familiarize yourself with question formats.
- Break problems into smaller steps: Approach complex problems systematically, focusing on one step at a time.
- Review mistakes: Analyze any errors you make and ensure you understand why the correct answers work.
By following these strategies, you can make your study sessions more effective and increase your chances of performing well. The key is consistency and a balanced approach to both learning and practicing.
Mastering the Periodic Table in Chapter 7
Understanding the organization of elements is essential for grasping the relationships between different substances and predicting their behavior. The periodic table is a powerful tool that reveals patterns and trends in the properties of elements. Mastering its structure helps in recognizing how elements interact and how their characteristics change across periods and groups.
To gain a deep understanding of the periodic table, focus on the following key areas:
- Element Groups: The table is organized into columns called groups. Elements in the same group share similar properties, such as reactivity or atomic structure. Learn the characteristics of each group, including alkali metals, halogens, and noble gases.
- Periodic Trends: As you move across periods (rows) or down groups, certain trends emerge. These include electronegativity, ionization energy, and atomic radius. Understanding these trends helps predict the behavior of elements in reactions.
- Block Classification: Elements are also grouped into blocks based on their electron configurations. These include s, p, d, and f blocks. Each block has distinct properties and trends that are important for understanding element reactivity.
By regularly reviewing the periodic table and practicing with real-world examples, you’ll be able to apply this knowledge to solve complex problems and understand the fundamental behavior of elements. Building this foundation is essential for progressing in the subject.
Understanding the Mole Concept for Success
One of the foundational ideas in the study of matter is understanding how to quantify and relate the amount of substance. The concept of counting particles at the microscopic level is essential for solving many problems in the field. This understanding allows you to move from theoretical concepts to practical applications in various scientific calculations.
Why the Mole Concept Matters
The mole concept bridges the gap between the atomic scale and the macroscopic scale. It helps in determining how many atoms, molecules, or ions are present in a given sample of substance. By using the mole as a unit, you can express large numbers of particles in a manageable form.
Key Elements of the Mole Concept
- Avogadro’s Number: The number of particles in one mole of a substance, approximately 6.022 × 10²³. This value is central to understanding the mole concept and performing calculations.
- Molar Mass: The mass of one mole of a substance, typically measured in grams per mole (g/mol). This value is crucial for converting between the mass of a substance and the number of moles.
- Stoichiometry: This is the process of using mole relationships to determine the proportions of reactants and products in a chemical reaction. It is essential for making accurate calculations in reactions.
Mastering the mole concept allows you to approach scientific problems with confidence, as it is an indispensable tool for both quantitative and qualitative analysis. Practicing these calculations and understanding their real-world applications will lead to greater success in scientific assessments.
Balancing Chemical Reactions Made Simple
Balancing reactions is a crucial skill in understanding how substances interact. The process ensures that the law of conservation of mass is followed, meaning the same number of atoms must appear on both sides of the equation. While it may seem complex at first, following a few simple steps can make balancing equations more manageable and even intuitive.
Step-by-Step Approach
Start by writing the unbalanced equation with all reactants and products correctly identified. Then, follow these guidelines to achieve a balanced equation:
- Count the atoms: Begin by counting the number of atoms of each element on both sides of the equation.
- Adjust coefficients: Use coefficients to balance the number of atoms for each element. Start with the most complex molecule and adjust one element at a time.
- Double-check: After adjusting the coefficients, go back and verify that the number of atoms for each element is the same on both sides.
Common Tips and Tricks
- Balance hydrogen and oxygen last: These elements are often part of multiple compounds, making them more difficult to balance early on.
- Use fractions if needed: If you are stuck with uneven numbers of atoms, fractions can be used to balance equations. Just multiply the entire equation by the denominator to remove the fraction.
- Practice: The more equations you balance, the quicker and more intuitive the process becomes. Regular practice is key to mastering this skill.
By following these steps and practicing consistently, balancing reactions will become a much simpler task. Understanding the logic behind the process will allow you to solve even the most challenging equations with ease.
Interpreting Chemical Symbols and Formulas
Understanding the language of molecules is fundamental in scientific studies. Chemical symbols and formulas provide a concise way to represent elements and compounds, revealing important details about their composition and structure. Decoding these symbols allows you to grasp the underlying relationships between substances and predict their behavior in various reactions.
Breaking Down Chemical Symbols
A chemical symbol is a shorthand way of representing an element. Each element is assigned a unique symbol, typically derived from its Latin name or an abbreviation of its English name. Here’s how to interpret them:
- Element symbols: Each element has a one- or two-letter symbol. The first letter is capitalized, while the second letter, if present, is lowercase (e.g., H for hydrogen, O for oxygen, Na for sodium).
- Atomic number: The atomic number, found on the periodic table, tells you how many protons are in the nucleus of each atom of that element. It also helps determine the element’s position in the table.
- Atomic mass: The atomic mass is the average mass of the atoms of an element, accounting for isotopes. This is typically found beneath the element symbol.
Understanding Chemical Formulas
A chemical formula represents the types and quantities of atoms in a compound. It is crucial for understanding the proportions in which elements combine to form a substance. Here are the key components:
- Subscripts: Numbers written below and to the right of an element symbol represent the number of atoms of that element in a molecule (e.g., H₂O indicates two hydrogen atoms and one oxygen atom).
- Coefficients: A number in front of a chemical formula indicates the number of molecules or moles present (e.g., 2H₂O means two molecules of water).
- Polyatomic ions: Some formulas include groups of atoms that act as a unit. These are typically enclosed in parentheses and carry a subscript (e.g., Ca(OH)₂ indicates one calcium atom, two oxygen atoms, and two hydrogen atoms in the compound).
Once you understand how to read and interpret symbols and formulas, you can gain a deeper understanding of the relationships between substances and their roles in various processes. Practice recognizing these patterns will enhance your ability to solve complex problems and predict outcomes accurately.
How to Tackle Multiple-Choice Questions
Multiple-choice questions often test your understanding of key concepts and your ability to apply knowledge quickly and accurately. While these questions may seem straightforward, they can sometimes be tricky. Developing a systematic approach to answering them can improve your accuracy and confidence during assessments.
Step-by-Step Strategy
To tackle multiple-choice questions effectively, follow these steps:
- Read the question carefully: Take a moment to understand exactly what the question is asking before looking at the options. This will help you focus on what is important and avoid misinterpreting the problem.
- Eliminate obviously wrong answers: Rule out choices that are clearly incorrect. This increases your chances of selecting the correct answer if you need to guess.
- Look for clues in the question: Often, the phrasing of the question or other options can give you hints. Pay attention to keywords that stand out and guide you toward the correct choice.
- Consider all options: Avoid jumping to conclusions. Even if one option looks correct, check all the choices before making your final decision.
Common Pitfalls to Avoid
Here are some common mistakes to be aware of when answering multiple-choice questions:
- Overlooking negative phrasing: Questions with words like “not,” “except,” or “none” can easily mislead you. Always focus on what the question is asking rather than assuming the most obvious answer.
- Choosing the first option that seems right: Sometimes the first choice looks correct, but it’s important to evaluate all available options before deciding.
- Overthinking: Don’t second-guess yourself too much. Often, your first instinct is correct, especially if you have studied the material thoroughly.
Example Question Breakdown
Let’s look at an example to illustrate how these strategies work:
| Question | Options |
|---|---|
| Which of the following is a correct description of the process of photosynthesis? | A) The process by which plants absorb water B) The process of converting light energy into chemical energy C) The breakdown of food into smaller molecules D) The production of oxygen from the soil |
In this case, by eliminating options A, C, and D, you can easily conclude that option B is the correct answer. This strategy ensures that you approach every multiple-choice question with logic and focus.
By practicing these techniques, you can improve your ability to answer multiple-choice questions efficiently and effectively, ensuring better results during evaluations.
Problem-Solving Strategies for Chemistry Tests
Approaching complex problems during assessments requires a clear strategy to break down the steps and avoid feeling overwhelmed. The key to successful problem-solving is understanding the underlying principles and applying logical methods to find solutions. Developing an effective approach can make even the most difficult questions more manageable.
Step-by-Step Approach to Solving Problems
To tackle problems methodically, follow these essential steps:
- Read the question carefully: Start by understanding what is being asked. Pay attention to any details or conditions specified in the problem.
- Identify known and unknown variables: List out the information provided in the problem and what you are required to find. This helps in visualizing the path to the solution.
- Choose the right formula or concept: Depending on the type of problem, select the appropriate formula or principle that will help you link the known and unknown variables.
- Perform the calculations step-by-step: Break down the solution into manageable parts. Solve each step carefully and check for errors along the way.
- Review the solution: Once you have a solution, check if it makes sense in the context of the problem. Verify your calculations and ensure that your answer is reasonable.
Common Mistakes to Avoid
Avoiding common errors can greatly improve your efficiency in solving problems. Here are some pitfalls to be mindful of:
- Skipping units: Always keep track of units during calculations. Make sure to convert them where necessary to maintain consistency and accuracy.
- Rushing through calculations: Take the time to work through each step carefully. Rushing can lead to careless mistakes.
- Not double-checking the final answer: Always review your work to ensure that the final answer is both correct and appropriate for the question posed.
Example Problem Breakdown

Let’s look at a simple example to demonstrate the problem-solving process:
| Question | Given Information | Solution Steps |
|---|---|---|
| What is the molar mass of NaCl? | Na: 23 g/mol Cl: 35.5 g/mol |
Step 1: Add the atomic masses of Na and Cl. Step 2: 23 + 35.5 = 58.5 g/mol (Molar mass of NaCl) |
By applying the steps systematically, this problem becomes straightforward. With consistent practice, you can solve more complex problems in the same manner.
By following these strategies and avoiding common mistakes, you can improve your problem-solving skills and perform confidently during assessments.
Explaining the Law of Conservation of Mass
The principle that matter is neither created nor destroyed during a chemical reaction is fundamental in understanding how substances interact. This concept ensures that the total mass of the reactants before a reaction is equal to the total mass of the products after the reaction. The idea that matter remains constant, even though it may change form, is crucial for interpreting the outcomes of chemical processes.
According to this law, no matter what type of reaction occurs–whether it’s a synthesis, decomposition, or combustion–the amount of matter stays unchanged. While atoms may rearrange to form new substances, the total mass remains the same. This is why balancing reactions is an essential part of studying these processes, as it allows us to see that all mass is accounted for on both sides of the equation.
Applications of the Law
The law has many practical applications in fields like industry, laboratory experiments, and even environmental studies. Understanding the conservation of mass helps in predicting the outcome of reactions and managing resources more efficiently. Here are a few examples of how this principle is applied:
- Manufacturing Processes: In industries, the law is used to ensure that materials are used efficiently without wastage. By keeping track of reactant and product masses, manufacturers can optimize their production processes.
- Environmental Impact: The principle also plays a key role in understanding the impact of chemical processes on the environment. For instance, in waste treatment or pollution control, the total mass of the substances involved remains the same, which helps scientists track contaminants and design effective solutions.
- Quantitative Analysis: In chemical laboratories, the law is essential for determining the exact amounts of reactants needed to produce a desired product. It ensures that experiments are carried out with precision and reliability.
Practical Example
Consider the reaction between hydrogen and oxygen to form water:
2H2 + O2 → 2H2O
Before the reaction, the total mass of hydrogen and oxygen is equal to the mass of the water produced after the reaction. This simple example demonstrates how the mass remains conserved throughout the process.
In conclusion, the Law of Conservation of Mass is a vital concept that underpins our understanding of how matter behaves during chemical reactions. It assures us that no matter is lost or gained, providing a predictable and stable foundation for scientific experimentation and real-world applications.
How to Use Stoichiometry Effectively
Stoichiometry is an essential tool in understanding the relationships between the quantities of reactants and products in chemical reactions. By using this method, you can predict how much of each substance will be consumed or produced in a given reaction. The key to mastering stoichiometry lies in the ability to convert between units, balance equations, and apply the correct mole ratios. With practice, this technique can be used to solve a variety of problems involving chemical reactions, from simple conversions to more complex analyses.
To apply stoichiometry effectively, it’s crucial to follow a structured approach. The general steps involve identifying the known quantities, writing the balanced equation, and using conversion factors to calculate the unknown quantities. By carefully analyzing the given information and systematically applying the stoichiometric method, you can easily solve for missing values in most chemical equations.
Steps to Use Stoichiometry
Here is a simple guide to follow when using stoichiometry:
- Balance the equation: Make sure the chemical equation is balanced, meaning the number of atoms of each element is the same on both sides of the equation.
- Convert units to moles: If the given data is in units other than moles (e.g., grams, liters), convert it to moles using the appropriate molar mass or volume.
- Use mole ratios: From the balanced equation, use the mole ratios to relate the quantities of the reactants and products.
- Convert moles back to desired units: Once you have the number of moles of the unknown substance, convert it to the required units (grams, liters, molecules, etc.).
Example Problem

Let’s consider a simple example to see how stoichiometry works in practice:
Suppose you are given 10 grams of oxygen and need to determine how many grams of water will be produced from this quantity. The balanced equation for the reaction between hydrogen and oxygen to form water is:
2H2 + O2 → 2H2O
To solve this, follow these steps:
- Step 1: Convert the given mass of oxygen to moles using its molar mass (32 g/mol).
- Step 2: Use the mole ratio from the balanced equation (1 mole O2 : 2 moles H2O) to determine the number of moles of water.
- Step 3: Convert moles of water to grams using the molar mass of water (18 g/mol).
Following these steps, you would calculate that approximately 5.6 grams of water will be produced from 10 grams of oxygen.
By mastering stoichiometry and practicing these steps, you’ll be able to tackle a wide range of problems with confidence and precision.
Understanding the Role of Catalysts
Catalysts are substances that play a crucial role in speeding up chemical reactions without being consumed in the process. These compounds lower the activation energy required for a reaction to take place, allowing reactions to occur more efficiently and at a faster rate. Whether in industrial processes or biological systems, catalysts are essential for making many chemical transformations possible under conditions that would otherwise be too slow or impractical.
In essence, catalysts provide an alternative pathway for reactions, making it easier for reactants to transform into products. This is particularly important in reactions that need to occur under specific conditions, such as those requiring high temperatures or pressures. By offering a more favorable route for the reaction, catalysts help in achieving desired outcomes with greater energy efficiency and less waste production.
How Catalysts Work

The primary mechanism by which catalysts function is by lowering the activation energy. The activation energy is the minimum amount of energy that the reactants must possess for the reaction to occur. Without a catalyst, the reactants must collide with enough energy to break bonds and form new ones. However, a catalyst facilitates the breaking and making of bonds by providing a surface or an environment where reactants can come together more easily.
- Surface catalysis: Many catalysts work by providing a surface for reactants to bind to, which increases the likelihood of collisions and helps stabilize intermediate compounds formed during the reaction.
- Enzyme catalysis: In biological systems, catalysts known as enzymes speed up reactions by binding to specific molecules and lowering the energy needed to complete the reaction.
Examples of Catalysis in Action
Catalysis is used in a wide variety of applications, from the production of fuels to the breakdown of pollutants. Here are a few examples:
- Industrial processes: Catalysts are used in refining petroleum to produce fuels and other chemicals. For example, platinum is used in catalytic converters in cars to reduce harmful emissions.
- Biological processes: In the human body, enzymes such as amylase help break down food, making digestion faster and more efficient.
- Environmental impact: Catalysts are crucial in reducing pollutants in the air and water, such as in the catalytic converters of vehicles and in wastewater treatment plants.
By facilitating these processes, catalysts enable faster reactions and reduce the need for excessive energy inputs, making chemical processes more sustainable and effective. Understanding how catalysts work is key to harnessing their power in various scientific and industrial fields.
Reviewing Unit Conversions for Accuracy
Accurate unit conversions are vital in scientific calculations, as they allow for consistent results across various systems of measurement. In many situations, converting between units is necessary to ensure that calculations are correct and comparable. Whether working with mass, volume, temperature, or other physical quantities, understanding how to properly convert units is essential for precise and reliable outcomes.
Unit conversion is a straightforward process, but it can become complex when dealing with different scales or when applying conversion factors incorrectly. To avoid errors, it’s important to follow a systematic approach and double-check each step of the conversion. By focusing on the correct relationships between units and using the right conversion factors, the chances of making mistakes can be minimized.
Steps for Accurate Unit Conversion
Here are the basic steps to follow when performing unit conversions:
- Identify the starting unit and the target unit: The first step is to clearly determine the units involved in the conversion. For example, converting from grams to kilograms or from miles to kilometers.
- Determine the conversion factor: This is the numerical ratio that allows you to convert one unit into another. Make sure the factor is accurate and appropriate for the units in question.
- Multiply by the conversion factor: Use multiplication or division based on the conversion factor to change the units. For instance, 1 kilogram is equal to 1000 grams, so multiplying the given value by this ratio will convert it.
- Check the result: Finally, verify that the converted value makes sense in the context of the problem, ensuring that both the magnitude and units are correct.
Common Unit Conversion Mistakes to Avoid
Even though unit conversions are essential for accuracy, there are some common pitfalls to watch out for:
- Incorrect conversion factor: Always make sure you are using the correct factor. Mixing up conversion ratios can lead to significant errors in calculations.
- Forgetting to cancel units: It’s easy to overlook the cancellation of units in a conversion. Remember that units should cancel out as part of the process, leaving you with the desired unit.
- Inconsistent units in multi-step conversions: If your conversion involves multiple steps, ensure that each intermediate unit is properly converted before proceeding to the next step.
- Rounding too early: It’s tempting to round off numbers early in the calculation process. However, rounding too soon can lead to cumulative errors, so it’s best to keep extra significant figures throughout the calculation and round at the end.
By following these steps and being mindful of common mistakes, you can ensure accurate unit conversions in any scientific or mathematical context. The more practice you get with conversions, the more intuitive and error-free the process will become.
Key Chemical Properties You Should Know
Understanding the essential characteristics of substances is fundamental for predicting how they will behave in various reactions. Chemical properties provide insight into how substances interact with each other under specific conditions. These properties are crucial for anyone working with materials, whether in scientific experiments or industrial applications. Recognizing these characteristics allows you to anticipate the outcomes of reactions and handle substances safely and effectively.
Some key chemical properties to be familiar with include reactivity, acidity or basicity, oxidation states, and the ability to form bonds. Each of these properties plays a critical role in how substances react and combine. By understanding them, you can better control and manipulate reactions to achieve the desired results.
Important Chemical Properties
Here are several key properties that you should know:
| Property | Description | Example |
|---|---|---|
| Reactivity | How readily a substance reacts with other substances, such as oxygen, acids, or bases. | Iron rusting when exposed to oxygen in the air. |
| Acidity or Basicity | The measure of a substance’s ability to donate protons (acids) or accept protons (bases). | Hydrochloric acid (HCl) as an acid, and sodium hydroxide (NaOH) as a base. |
| Oxidation State | The charge of an atom in a compound, which can affect its reactivity. | Iron (Fe) in Fe2O3 has an oxidation state of +3. |
| Flammability | The ability of a substance to catch fire and combust in the presence of heat and oxygen. | Gasoline is highly flammable and can ignite easily. |
| Electronegativity | The tendency of an atom to attract electrons when it forms a bond. | Fluorine has a high electronegativity, meaning it strongly attracts electrons in chemical bonds. |
Applications of Chemical Properties
Understanding these properties allows you to predict how substances will behave in reactions, which is particularly useful in many fields. For example, in pharmaceuticals, the reactivity of compounds is crucial for drug formulation, while in manufacturing, the flammability and acidity of materials determine their suitability for different processes. Additionally, knowing the oxidation states of elements helps in the creation of alloys or the extraction of metals.
Being well-versed in these key properties not only enhances your understanding of materials and reactions but also helps you make informed decisions when handling substances in both everyday and specialized situations.
How to Approach Exam Anxiety
Facing an exam can be a stressful experience, especially when the material feels overwhelming or challenging. However, managing anxiety is key to performing well. By adopting the right strategies, you can remain calm, focused, and prepared, which will help you approach the situation with confidence. Understanding how to handle stress before and during the assessment can make a significant difference in your overall performance.
Preparation is Key

One of the best ways to reduce anxiety is through thorough preparation. The more familiar you are with the content, the less intimidating it will seem. Here are a few strategies that can help:
- Break down the material: Rather than trying to learn everything in one sitting, divide the material into smaller, manageable sections.
- Practice regularly: Consistent practice reinforces your knowledge and builds confidence.
- Utilize various resources: Supplement your study with practice questions, summaries, and study guides to reinforce key concepts.
During the Exam

Even when you have prepared well, the pressure of the exam setting can still trigger anxiety. Here are some tips for managing stress during the exam:
- Stay calm: If you feel overwhelmed, take a few deep breaths to center yourself. Staying calm will help you think more clearly.
- Read instructions carefully: Ensure you understand the requirements before jumping into answering questions.
- Manage your time: Pace yourself throughout the exam to ensure that you have enough time to complete all the questions.
By focusing on preparation and maintaining a calm demeanor, you can minimize anxiety and set yourself up for success. It’s important to remember that taking care of your mental well-being is just as important as studying the material itself.