1994 AP Chemistry Free Response Answers Explained

When preparing for a challenging academic test, understanding how to approach difficult questions is essential. The ability to tackle intricate problems not only boosts confidence but also helps to demonstrate a deeper grasp of the material. This section explores strategies and techniques for solving advanced tasks, offering insight into how to approach various types of queries effectively.
Understanding the structure and requirements of such exercises is the first step towards success. Many of these tasks involve multiple stages, requiring both knowledge and critical thinking. It’s important to be prepared for the complexity and the need to explain concepts clearly and concisely. With a focused approach, you can navigate even the toughest parts of an exam.
Throughout this guide, we will break down the steps for tackling these questions and highlight key strategies for providing precise, well-structured solutions. By examining previous sets of tasks and solutions, you’ll gain practical experience in solving problems, allowing you to refine your techniques for future assessments.
1994 AP Chemistry Free Response Overview
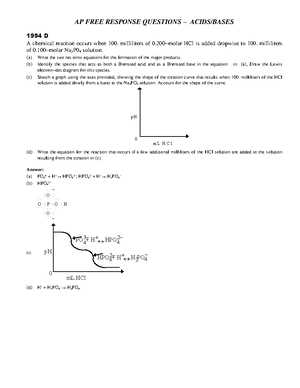
In this section, we will explore the structure and key elements of a set of complex exam questions that test a student’s ability to apply scientific principles in practical scenarios. These types of tasks are designed to challenge not only your factual knowledge but also your critical thinking and problem-solving skills. The goal is to assess your ability to analyze, interpret, and solve advanced problems in a structured and logical manner.
Key Areas Covered
The questions in this segment focus on core concepts and applications that require a deeper understanding of scientific principles. They span a variety of topics, including but not limited to reaction mechanisms, equilibrium processes, and quantitative calculations. The aim is to provide a comprehensive understanding of the subject while honing skills in logical reasoning and precise articulation of solutions.
Skills and Techniques Required
To perform well in these types of questions, it is essential to combine theoretical knowledge with practical problem-solving techniques. Each question is an opportunity to demonstrate how well you can apply your learning to new situations. Critical thinking, clear writing, and careful organization of your answers are crucial for success. Mastering these aspects will allow you to approach each problem with confidence and accuracy.
Understanding the AP Chemistry Exam Format
The format of a rigorous academic exam plays a crucial role in preparing effectively. Knowing the structure and expectations of the test allows you to approach it with a clear strategy and confidence. The exam is divided into two main sections: multiple-choice questions and a set of tasks requiring detailed written explanations. Each section is designed to evaluate different aspects of your knowledge and reasoning abilities.
Multiple-choice questions assess your ability to recall facts and apply concepts in a variety of scenarios. These questions are designed to test both your basic understanding and your ability to think critically under time constraints. On the other hand, the section involving written solutions demands a deeper level of comprehension, as it requires you to explain complex processes and provide reasoned, step-by-step solutions.
Familiarizing yourself with the format and practicing under timed conditions is key to performing well on the exam. This not only helps you manage your time but also ensures that you can approach each question with the necessary focus and detail. By understanding the expectations of both sections, you will be better equipped to demonstrate your knowledge effectively during the test.
Importance of Free Response Questions
Open-ended tasks on an exam play a critical role in assessing your ability to demonstrate comprehensive understanding and articulate complex ideas. These questions go beyond simple recall, testing your capacity to apply learned concepts in unfamiliar situations. They are designed to measure both your depth of knowledge and your ability to express your reasoning clearly and logically.
These types of questions require more than just the correct answer; they evaluate how well you can explain and justify your thought process. Being able to break down a complex problem into manageable steps, while clearly communicating your approach, is essential for success. They also provide an opportunity to showcase your critical thinking, organization, and written communication skills, which are invaluable in both academic and professional settings.
Mastering this section of the exam is vital for scoring well, as it accounts for a significant portion of your total score. By preparing thoroughly and practicing how to structure your responses effectively, you can increase your chances of demonstrating mastery over the subject matter and earning maximum points.
How to Approach the 1994 Questions
When tackling complex exam questions, it is important to have a clear strategy that helps you break down each problem systematically. These tasks often require both analytical thinking and precise communication. By following a structured approach, you can improve your ability to provide complete and accurate solutions.
- Read each question carefully: Before jumping into the solution, take time to understand exactly what is being asked. Pay attention to key terms, units, and any specific instructions that might guide your approach.
- Organize your thoughts: Break the question down into smaller, more manageable parts. Identify the key concepts and processes that need to be addressed, and structure your answer logically.
- Plan your approach: Outline the steps you will take to solve the problem before writing anything down. This will help you avoid unnecessary mistakes and ensure that your reasoning is clear and well-organized.
- Show all work: Even if you know the answer, it’s essential to demonstrate your thought process. Include intermediate steps, calculations, and explanations to support your solution.
- Double-check your solution: After completing the task, review your work to ensure that all aspects of the question have been addressed and that your reasoning is sound.
By following these steps, you can approach even the most challenging questions with confidence, ensuring that your solutions are clear, logical, and well-supported by evidence.
Key Topics in 1994 Free Response
In any rigorous exam, certain subjects tend to dominate the set of tasks, reflecting their importance in understanding the fundamental principles of the field. The topics featured in these types of questions often require a combination of theoretical knowledge and practical application. Recognizing the key areas covered will help you focus your preparation and develop a stronger grasp of the material.
Commonly tested areas include reaction mechanisms, thermodynamics, and kinetics. Understanding how chemical processes occur at the molecular level and being able to predict their outcomes is crucial. Additionally, topics like equilibrium calculations and acid-base reactions are frequently explored, requiring a solid understanding of both qualitative and quantitative aspects.
Mastering these core concepts and practicing related problems will help you tackle the most challenging tasks with confidence, ensuring that you are well-prepared for any question related to these essential topics.
Common Mistakes to Avoid
When tackling complex exam questions, it’s easy to make errors that can significantly affect your overall performance. Recognizing and avoiding these mistakes is key to improving your results and ensuring that your solutions are both accurate and well-structured. Many of these errors stem from rushed work, misinterpretations, or a lack of attention to detail.
A common mistake is failing to fully read and understand the question before beginning your solution. Often, crucial information or instructions are embedded in the question that may affect how you approach the problem. Additionally, neglecting to show all steps or explanations in your calculations can lead to lost points, even if the final result is correct.
Another frequent error is neglecting units and significant figures. Improper use of units or rounding errors can undermine the accuracy of your work. Similarly, overlooking key concepts such as assumptions in calculations or failing to address all parts of a question will limit the effectiveness of your response.
By being mindful of these common pitfalls and developing a careful, organized approach to each question, you can improve the quality of your answers and maximize your score.
Review of Chemical Reactions in 1994
Understanding the various types of chemical processes and reactions is essential for solving complex problems on an exam. Chemical transformations involve changes in the composition of substances and can take many forms, such as combustion, synthesis, or decomposition. Mastery of reaction mechanisms and the ability to predict outcomes are fundamental skills needed to approach related tasks effectively.
- Balancing equations: One of the key skills in understanding reactions is the ability to correctly balance chemical equations. This ensures that the law of conservation of mass is respected, and all reactants and products are accounted for in the correct proportions.
- Identifying reaction types: Being able to categorize reactions–whether they are redox, acid-base, or precipitation–helps in predicting products and determining the conditions under which reactions occur.
- Understanding stoichiometry: The relationship between reactants and products is crucial for calculating quantities involved in reactions. This includes understanding limiting reagents and calculating the theoretical yield of a reaction.
- Reaction rates and mechanisms: Knowing how to analyze the speed of reactions and how different factors like temperature and concentration affect them is vital. Mechanisms provide insight into the sequence of steps that occur during a reaction, giving a deeper understanding of the process.
By reviewing and practicing these core aspects of chemical reactions, you will be well-prepared to tackle related questions on the exam, applying your knowledge to both familiar and new scenarios with confidence.
Tips for Answering Complex Calculations
When faced with challenging calculations, a systematic approach is crucial for ensuring accuracy and clarity. These types of problems often require careful attention to detail, as well as a thorough understanding of the underlying concepts. By following a structured method, you can solve even the most difficult problems with confidence and precision.
- Start with a clear strategy: Before diving into the calculations, take a moment to identify the given information, what is being asked, and the relationships between the variables. This will help you understand the problem and determine the best approach.
- Organize your work: Break the calculation into smaller, more manageable steps. Write down each intermediate step clearly, making it easier to track your progress and spot any potential errors.
- Check units and conversions: Always pay attention to units throughout the calculation process. Ensure that all units are consistent and convert them as needed to avoid mistakes in the final result.
- Use appropriate significant figures: Be mindful of the precision required in your final answer. Use the correct number of significant figures based on the given data and follow the standard rules for rounding.
- Double-check your final result: After completing the calculations, revisit your solution to ensure that no steps were skipped or miscalculated. If possible, estimate the result to check its reasonableness.
By implementing these strategies, you will enhance your ability to tackle complex calculations and provide accurate, well-organized solutions that demonstrate your full understanding of the material.
Examining Chemical Equilibrium in 1994
Understanding the concept of equilibrium is fundamental for solving various problems related to reversible reactions. At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction, resulting in no net change in the concentrations of reactants and products. This dynamic balance is influenced by factors such as concentration, temperature, and pressure, making it essential to grasp how to manipulate these conditions for problem-solving.
The Equilibrium Constant
The equilibrium constant, often denoted as K, is a key parameter used to quantify the position of equilibrium. It represents the ratio of the concentrations of products to reactants at equilibrium, raised to the power of their respective stoichiometric coefficients. A high value of K indicates that the reaction favors the products, while a low value suggests that the reactants are favored.
Le Chatelier’s Principle
Le Chatelier’s Principle helps predict how a system at equilibrium will respond to external changes. If a system is disturbed by changing concentration, pressure, or temperature, the system will shift in a direction that counteracts the change. For example, increasing the concentration of a reactant will generally shift the equilibrium toward the products to restore balance.
Mastering the principles of equilibrium allows you to approach related questions with confidence, ensuring that you can analyze and manipulate systems effectively to predict their behavior under various conditions.
Strategies for Time Management
Effective time management is crucial when tackling an exam, especially when dealing with complex tasks that require both thoughtful analysis and detailed solutions. Without a clear strategy, it’s easy to get caught up in individual questions, leaving less time for the remaining ones. By using time management techniques, you can ensure that you allocate enough time for each task, reducing stress and maximizing performance.
- Understand the format: Before you begin, familiarize yourself with the structure of the exam. Knowing the number of questions and their relative difficulty will help you set a reasonable time limit for each section.
- Allocate time per question: Based on the total time available and the number of questions, estimate how much time you should spend on each task. It’s helpful to leave a few minutes at the end for reviewing your work.
- Prioritize easier questions: Start with the questions you find easiest. This will help you build confidence and ensure that you secure points quickly. If you get stuck on a difficult question, move on and come back to it later.
- Break down tasks: For more complex problems, break them into smaller, manageable steps. This will help you stay focused and avoid feeling overwhelmed by the overall complexity of the question.
- Keep track of time: As you work through the exam, be mindful of the time. Use a watch or the exam clock to monitor your progress, ensuring that you stay on track and don’t spend too much time on any one question.
By implementing these time management strategies, you can approach the exam more confidently, ensuring that you complete all tasks efficiently without rushing or sacrificing accuracy.
Grading and Scoring of Free Response

The grading and scoring of written tasks on an exam are designed to assess not only the correctness of your answers but also the clarity and logical structure of your reasoning. These tasks typically involve multiple steps, and each step is evaluated for accuracy and completeness. Understanding how your responses are graded will help you focus on key areas to maximize your score.
Evaluation Criteria
In general, graders look for well-organized, detailed solutions that clearly show your understanding of the concepts. Points are awarded for each correct step in the solution, with partial credit often given for intermediate steps, even if the final answer is incorrect. Accuracy is important, but the clarity of your explanations also plays a key role in how your response is scored.
How to Maximize Your Score
To improve your chances of scoring well, make sure to write out every step of your solution, showing how you arrived at each conclusion. Even if you are confident in your final answer, demonstrating the thought process and the reasoning behind each decision will help ensure you receive full or partial credit for your work. Additionally, paying attention to detail, such as using proper units and significant figures, can prevent losing points over small mistakes.
By understanding the grading system and focusing on presenting clear, logical, and accurate solutions, you can maximize your score on these written tasks.
Using the 1994 Answers for Study
Reviewing past exam solutions is an excellent way to prepare for upcoming tests, as it allows you to familiarize yourself with the structure, complexity, and types of questions that may be asked. By analyzing previous tasks and their solutions, you can gain insight into the expected approach, key concepts, and common pitfalls to avoid. This method helps reinforce your understanding and enables you to practice solving similar problems under timed conditions.
Benefits of Reviewing Past Solutions
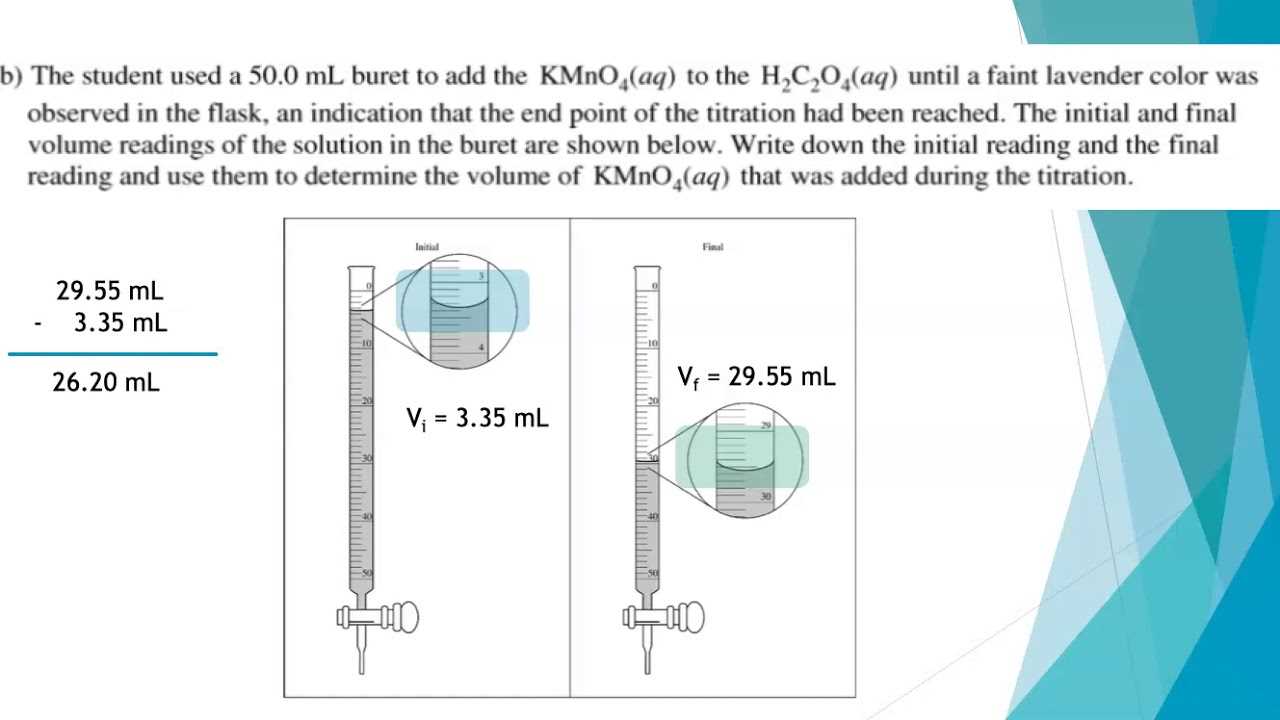
Studying past solutions offers several advantages. First, it helps you recognize patterns in the types of questions asked, allowing you to focus your study on the most commonly tested concepts. Second, by seeing how detailed, accurate solutions are presented, you can learn how to structure your own responses effectively. Finally, revisiting these problems helps build confidence as you practice solving them independently.
How to Use Past Solutions Effectively

To make the most of past solutions, try the following approaches:
- Work through the problems yourself: Before looking at the solutions, attempt to solve the problems on your own. This helps you identify areas where you may need further review or practice.
- Compare your solutions: After solving a problem, compare your answer with the provided solution. Pay close attention to how the solution is structured and whether you made any mistakes in your reasoning or calculations.
- Identify common themes: Take note of frequently tested concepts or question types. Focus your study on these areas to maximize your preparation.
Example Study Table
| Topic | Key Concept | Common Pitfall | Study Focus |
|---|---|---|---|
| Stoichiometry | Limiting reactants, mole ratios | Incorrect conversion between moles and grams | Practice mole-to-mole conversions, balancing equations |
| Acid-Base Equilibria | pH calculations, weak acids/bases | Ignoring the weak acid assumption | Focus on approximations for weak acids and bases |
| Thermodynamics | Enthalpy, entropy, Gibbs free energy | Forgetting to use correct signs for heat and work | Review calculation methods for ?G, ?H, and ?S |
By following these strategies and regularly reviewing previous solutions, you can improve both your conceptual understanding and your ability to apply that knowledge under exam conditions.
Understanding Acid-Base Equilibria in 1994

Acid-base equilibria are a critical area of study in many scientific fields, as they govern the behavior of substances in aqueous solutions. These systems involve reversible reactions between acids and bases, where the concentrations of reactants and products remain constant at equilibrium. The ability to understand and manipulate these reactions is essential for solving problems related to pH, buffer solutions, and titration curves.
In these equilibria, an acid donates protons (H+), while a base accepts them. The strength of an acid or base is determined by its ability to dissociate in water, with strong acids and bases dissociating completely and weak acids and bases dissociating partially. This dissociation is central to understanding pH calculations and the use of the equilibrium constant (K) to quantify the balance between reactants and products.
One important concept in acid-base equilibria is the relationship between the concentrations of the acid, its conjugate base, and the hydronium ion (H3O+). By applying the equilibrium expression, we can calculate the pH of a solution and determine whether it is acidic, neutral, or basic. The pH scale is logarithmic, meaning that even small changes in the concentration of hydrogen ions can lead to significant changes in pH.
Mastering the principles of acid-base equilibria allows for the accurate prediction of the outcomes of chemical reactions in a variety of contexts, from biological systems to industrial applications. Understanding how to approach these systems mathematically is key to solving related problems on exams and real-world scenarios.
Identifying Key Trends in AP Chemistry
In any advanced scientific field, recognizing patterns and trends is essential for solving complex problems efficiently. By understanding the recurring themes and concepts that appear across exams, students can focus their efforts on the most important topics and prepare more effectively. Identifying these trends helps in predicting the kinds of questions likely to be asked, allowing for targeted practice and deeper understanding.
Common Themes in Exam Questions
Across various exams, certain topics consistently emerge, often focusing on core concepts that underpin much of the subject. For example, equilibrium, thermodynamics, and kinetics are often emphasized in both theoretical and practical problem-solving scenarios. Additionally, questions related to bonding, molecular structure, and stoichiometry are regularly featured, requiring students to apply their knowledge of fundamental principles in diverse contexts.
Pattern Recognition in Problem Types
Another important aspect of exam preparation is recognizing the structure of common question types. Multiple-choice questions tend to assess conceptual understanding, while written tasks often require detailed, step-by-step reasoning and the application of complex formulas. By understanding how these questions are typically structured, students can allocate their time and effort more effectively during exam preparation.
By analyzing these patterns and trends, students can direct their study efforts more efficiently, improving their chances of performing well on both theoretical and practical exam sections. Focusing on key concepts, practicing problem-solving skills, and familiarizing themselves with common question types can help students gain confidence and mastery of the material.
Advanced Techniques for 1994 Solutions
Mastering complex problems requires more than just understanding fundamental concepts; it demands the application of advanced problem-solving techniques that can streamline your approach and ensure accuracy. By refining your approach to difficult questions, you can work more efficiently, reducing the chance of making simple errors and improving your overall performance. The key to success lies in knowing how to break down problems into manageable steps, applying sophisticated strategies, and recognizing underlying patterns that can simplify even the most challenging tasks.
Strategies for Efficient Problem Solving
There are several advanced techniques that can help improve the accuracy and speed of your solutions. The following methods are especially useful when facing complex problems:
- Dimensional Analysis: This technique helps you convert between units or determine unknown values by carefully tracking the units throughout the calculation. It ensures that all conversions are done systematically and reduces the likelihood of errors.
- Approximation Methods: In cases where exact calculations may be cumbersome, using approximations can simplify the problem without sacrificing accuracy. For example, applying the assumption of negligible values in equilibrium calculations can often provide a more straightforward solution.
- Use of Graphical Methods: Plotting data or using graphical representations (such as titration curves or reaction rates) can provide valuable insights and help you identify trends that might be missed in numerical calculations.
Improving Conceptual Understanding with Advanced Techniques
In addition to improving your technical skills, advanced techniques also focus on enhancing your conceptual understanding. By mastering the following approaches, you can develop a deeper insight into the subject matter:
- Utilizing Symmetry and Patterns: Many problems can be simplified by recognizing inherent symmetry in the system, such as balanced chemical equations or predictable reaction mechanisms. Identifying these patterns can help you predict outcomes more quickly and accurately.
- Integrating Multiple Concepts: Often, complex problems will require you to integrate knowledge from different areas of study, such as thermodynamics and kinetics. Strengthening your ability to combine concepts will make it easier to handle multifaceted problems.
- Critical Thinking and Reflection: Regularly reviewing your solutions and considering alternative methods for solving the same problem will help you refine your approach. This process encourages deeper understanding and prepares you for unfamiliar or novel questions.
By implementing these advanced techniques, you can approach difficult problems with greater confidence and efficiency, increasing your chances of success in both theoretical and applied questions. Mastering these methods is key to excelling in complex scenarios, allowing you to navigate through even the most challenging tasks with ease.
How to Improve Your Free Response Skills

To excel in exams that require detailed written answers, it’s essential to develop clear and concise problem-solving skills. These types of questions often test your ability to think critically, apply learned concepts, and explain your reasoning in a structured manner. Improving your ability to tackle these questions involves practicing specific strategies, refining your understanding of key topics, and learning how to effectively communicate your solutions.
Essential Strategies for Improvement

There are several effective techniques to improve your performance when faced with complex, open-ended problems:
- Practice with Past Questions: Regularly working through previous exam questions helps you familiarize yourself with the structure and complexity of the problems. It also allows you to practice formulating structured answers under timed conditions.
- Develop Step-by-Step Solutions: When solving a problem, break it down into clear, manageable steps. This not only makes your work more organized but also helps ensure you don’t miss any important details in the process.
- Focus on Clarity: Avoid overly complicated language or unnecessary jargon. The goal is to communicate your reasoning and conclusions clearly. Strive to be as concise and direct as possible while still providing all necessary explanations.
- Time Management: Allocate your time wisely during the exam. Set aside enough time to approach each problem carefully, but also be mindful of time limits to ensure you can complete all questions.
Key Areas for Review and Practice
In addition to general strategies, it’s important to review specific topics that are commonly tested in these types of questions. Focus on the following areas to improve your ability to tackle complex problems:
- Conceptual Understanding: Make sure you have a strong grasp of fundamental concepts. For example, understanding core principles like thermodynamics, acid-base equilibria, and stoichiometry will make it easier to approach various types of questions.
- Problem-Solving Skills: Focus on building your ability to approach problems systematically. This includes mastering the use of appropriate formulas, manipulating equations, and applying logical reasoning to reach correct conclusions.
- Effective Explanation: Practice explaining your thought process and solutions clearly. Whether writing out solutions on paper or in a study group, the ability to articulate your reasoning helps you organize your thoughts and strengthens your overall approach.
By applying these strategies and consistently practicing complex problems, you’ll enhance both your problem-solving abilities and your ability to communicate your solutions effectively, setting you up for success in any examination that requires detailed written answers.
Linking Past Exams to Current Study
One of the most effective ways to enhance your preparation for upcoming exams is to connect your current study material with past exam questions. By reviewing and analyzing previous exams, you can identify recurring themes and areas that are frequently tested. This strategy not only helps reinforce key concepts but also allows you to develop a deeper understanding of how to apply your knowledge in a testing environment.
Incorporating past exam questions into your current study routine serves multiple purposes. It helps you gauge the difficulty and scope of the topics being tested, familiarize yourself with the question format, and develop strong time-management skills. Furthermore, it highlights gaps in your understanding, allowing you to focus on areas that need further review or clarification.
When linking past exams to your ongoing study efforts, it’s crucial to focus on both conceptual knowledge and problem-solving skills. The following table outlines some common strategies for incorporating previous exams into your preparation:
| Strategy | Description | Benefit |
|---|---|---|
| Review Past Questions | Look through previous exams to identify common topics and question types. | Helps recognize recurring themes and prioritize study areas. |
| Simulate Exam Conditions | Practice solving past exam questions under timed conditions. | Improves time management and builds exam confidence. |
| Analyze Mistakes | Carefully review incorrect answers to understand mistakes and correct them. | Strengthens understanding and prevents similar errors in future exams. |
| Focus on Weak Areas | Identify the sections you struggled with in past exams and dedicate additional study time to those topics. | Enhances knowledge in areas of difficulty. |
By linking past exams to your current study materials, you can fine-tune your understanding, improve problem-solving techniques, and better prepare for future exams. This method also allows you to gauge your progress and adapt your study strategies based on your performance over time.
Maximizing Your AP Exam Score
Achieving the highest score on an Advanced Placement exam requires careful preparation, disciplined practice, and the ability to apply concepts efficiently during the test. To excel, it’s not only important to understand the material, but also to develop strategies that allow you to tackle a variety of questions under timed conditions. A focused study plan combined with smart test-taking techniques will significantly increase your chances of success.
Effective Study Techniques for Success
To maximize your performance, it’s essential to have a clear and efficient study strategy. Here are several effective methods to help you prepare:
- Build a Strong Foundation: Focus on mastering key concepts and principles that are central to the exam. These include topics like molecular structure, kinetics, and thermodynamics.
- Practice with Past Papers: Regularly solve problems from previous exams to familiarize yourself with the question formats and the types of challenges you will face.
- Review and Reinforce Weak Areas: Identify any areas where you struggle and devote extra time to those subjects. This ensures you don’t leave any gaps in your knowledge.
- Utilize Practice Problems: Solve as many practice problems as possible to improve problem-solving speed and accuracy.
Smart Strategies for the Exam Day
Once you’re ready, applying the right strategies on test day can make a significant difference in your performance. Here are some tips to help you maximize your score during the exam:
- Read Carefully: Make sure you understand the question before jumping into an answer. Pay attention to detail, and look for clues within the question.
- Manage Your Time: Be conscious of the time limit for each section. If you get stuck on a problem, move on and return to it later.
- Show Your Work: Even if you’re unsure of an answer, writing out your thought process may earn you partial credit for the steps you took toward solving the problem.
- Stay Calm and Focused: Keep your composure throughout the exam. If you feel stressed, take a moment to breathe and refocus.
The following table summarizes some of the key strategies to help you perform at your best:
| Strategy | Description | Benefit |
|---|---|---|
| Master Core Concepts | Understand the foundational principles and key topics. | Improves your ability to approach a wide variety of questions confidently. |
| Practice with Past Papers | Work through problems from previous exams to become familiar with the test format. | Reduces anxiety and increases comfort with the test structure. |
| Identify Weak Areas | Focus on areas where you need improvement. | Helps to strengthen your overall understanding and avoid common mistakes. |
| Time Management |