Chapter 26 Chemistry in the Environment Study Guide Answers
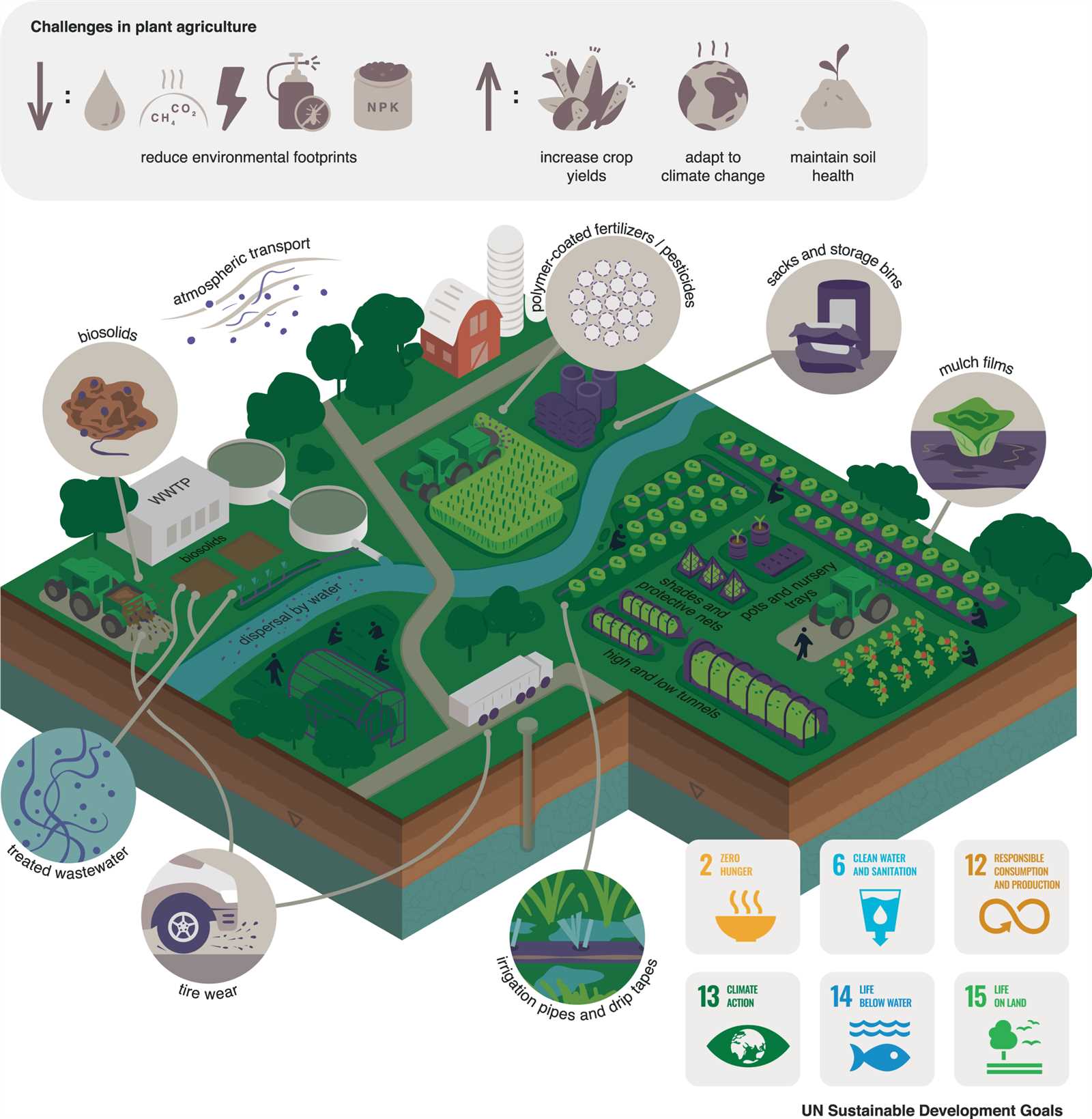
In this section, we explore the intricate relationships between natural systems and the substances that interact within them. The way elements and compounds circulate through various processes shapes the health and sustainability of ecosystems. This understanding is crucial for addressing current global challenges such as climate shifts and resource depletion.
Key interactions between human activity and natural processes are examined, highlighting how industrialization and modern practices affect air, water, and soil quality. Solutions to minimize negative impacts are considered through innovative techniques and scientific advancements aimed at restoring balance.
Exploring fundamental principles that govern these processes enables a deeper appreciation of how daily actions influence broader ecological outcomes. From natural cycles to modern-day challenges, this section provides a comprehensive look at pivotal concepts and their real-world implications.
Chapter 26 Chemistry in the Environment
This section focuses on how substances interact with natural systems and the fundamental processes that govern their behavior. These interactions play a vital role in maintaining balance within ecosystems and determining the sustainability of our planet’s resources. By understanding these relationships, we can better address pressing challenges such as pollution, climate change, and resource management.
Natural Cycles and Human Influence
Natural cycles, such as water, carbon, and nitrogen cycles, are crucial for maintaining ecological equilibrium. However, human activities have introduced significant alterations to these cycles, often leading to harmful effects. Industrial emissions, agricultural practices, and deforestation are just a few examples of how human actions disrupt these delicate systems, causing shifts in global temperatures, weather patterns, and biodiversity.
Scientific Approaches to Mitigation
To reverse or mitigate these negative effects, scientists have developed various strategies aimed at restoring balance. These include renewable energy technologies, waste reduction methods, and chemical solutions to clean polluted resources. The role of innovation and research is essential in finding sustainable alternatives that support both human needs and the health of natural systems.
Key Concepts in Environmental Chemistry
This section covers fundamental principles that explain how substances interact with natural systems and affect their functionality. Understanding these principles is essential for identifying how human activities influence global processes and what can be done to minimize negative impacts. Key ideas include how chemicals move through ecosystems, their reactions, and their effects on living organisms.
One critical concept is the balance between various elements and compounds in nature. The availability and movement of nutrients, gases, and pollutants determine the health of ecosystems and their ability to sustain life. By grasping these interactions, we can better understand the consequences of industrial emissions, waste disposal, and agricultural practices.
Another important concept is the role of renewable and non-renewable resources. As human society becomes more dependent on energy and raw materials, the need for sustainable solutions grows. The exploration of alternative energy sources, waste management, and pollution control is essential for reducing environmental strain and preserving natural resources for future generations.
Understanding Chemical Cycles in Nature
Nature is governed by a series of cycles that ensure the continuous movement and transformation of essential elements. These cycles maintain ecological balance by facilitating the recycling of matter within various ecosystems. By understanding how these cycles work, we can better appreciate their importance in sustaining life and how human activities may disrupt their natural flow.
Key Cycles in Nature
Several vital cycles are critical for maintaining the balance of life on Earth. Each cycle involves the movement of elements such as carbon, nitrogen, oxygen, and water through different stages of the biosphere. Below are some of the most significant cycles:
- Water Cycle – The movement of water through evaporation, condensation, precipitation, and infiltration ensures a continuous supply of water for all living organisms.
- Carbon Cycle – Carbon is cycled through the atmosphere, oceans, and living organisms, playing a key role in regulating temperature and supporting life processes.
- Nitrogen Cycle – Nitrogen moves between the atmosphere, soil, and organisms, where it is used to build proteins and other essential compounds.
- Oxygen Cycle – Oxygen is produced by plants during photosynthesis and consumed by organisms for respiration, creating a vital link for life processes.
Human Impact on Natural Cycles
Human activities can significantly alter the flow of these cycles, often leading to negative environmental effects. Some examples include:
- Deforestation, which disrupts the carbon and oxygen cycles by reducing the number of plants that absorb carbon dioxide and produce oxygen.
- Excessive use of fertilizers, which affects the nitrogen cycle by introducing too much nitrogen into the soil, leading to pollution and eutrophication of water bodies.
- Industrial emissions that release excess carbon dioxide into the atmosphere, contributing to global warming and disrupting the carbon cycle.
Understanding these cycles and their vulnerabilities is crucial for developing strategies to mitigate human impacts and restore balance to natural processes.
Impact of Pollution on Ecosystems
Pollution from human activities disrupts the natural balance of ecosystems, often leading to long-term environmental damage. Harmful substances, when introduced into air, water, and soil, alter vital processes that sustain life. These pollutants can have a wide range of negative effects, affecting both living organisms and the physical conditions of their habitats.
When toxic chemicals accumulate in natural habitats, they can poison species, disrupt reproduction, and reduce biodiversity. For example, heavy metals like mercury can accumulate in aquatic food chains, ultimately affecting fish populations and the animals that depend on them. Similarly, air pollutants such as sulfur dioxide and nitrogen oxides can lead to acid rain, which harms plant life, affects soil fertility, and degrades water quality.
Another significant impact is the disruption of nutrient cycles. Excessive use of fertilizers and industrial waste can introduce high levels of nitrogen and phosphorus into aquatic systems, leading to eutrophication. This process causes algae blooms that deplete oxygen levels in water, suffocating marine life and creating dead zones in oceans and lakes.
Furthermore, pollution can weaken ecosystems’ ability to adapt to changes, making it harder for species to survive in altered conditions. This ultimately leads to a loss of resilience in ecosystems, which is essential for maintaining biodiversity and ensuring ecological stability over time.
The Role of Greenhouse Gases

Greenhouse gases play a critical role in regulating the Earth’s temperature by trapping heat in the atmosphere. These gases naturally exist in the air, where they absorb and emit infrared radiation, helping to maintain a stable climate. However, human activities, especially the burning of fossil fuels, have increased their concentration, leading to enhanced warming and climate instability.
Types of Greenhouse Gases
There are several key gases that contribute to the greenhouse effect. Each of these gases varies in its ability to trap heat and its presence in the atmosphere. Below is a table that outlines the most common greenhouse gases and their characteristics:
| Gas | Source | Heat-Trapping Ability |
|---|---|---|
| Carbon Dioxide (CO2) | Burning of fossil fuels, deforestation | High |
| Methane (CH4) | Agricultural activities, livestock, landfills | Very High |
| Nitrous Oxide (N2O) | Agricultural processes, industrial activity | High |
| Water Vapor (H2O) | Natural processes, evaporation | Moderate |
| Ozone (O3) | Industrial emissions, vehicle exhaust | High |
Human Impact on Greenhouse Gas Levels
While greenhouse gases are naturally part of the Earth’s atmosphere, human activities have significantly increased their levels. The largest contributor to this increase is the burning of fossil fuels for energy, which releases carbon dioxide and methane into the atmosphere. Deforestation also reduces the planet’s ability to absorb CO2, further exacerbating the problem. As a result, the concentration of these gases has risen, intensifying the greenhouse effect and contributing to global warming.
Human Activities and Chemical Imbalance
Human actions have a profound effect on the natural balance of elements and compounds within ecosystems. Activities such as industrial production, agriculture, and urbanization introduce excess amounts of certain chemicals into natural systems, leading to disruptions in their natural cycles. These imbalances can result in long-term damage, affecting biodiversity, soil health, and air and water quality.
Pollution and Its Effects
One of the primary causes of chemical imbalance is pollution. Industrial emissions release harmful substances such as carbon dioxide, sulfur compounds, and nitrogen oxides into the air. Similarly, agricultural practices often involve the use of fertilizers and pesticides, which can contaminate soil and water systems. These pollutants can accumulate over time, overwhelming natural systems and causing negative effects on plant and animal life.
Excessive Resource Use
Another significant contributor to chemical imbalance is the overconsumption of natural resources. Overharvesting of fossil fuels, mining operations, and deforestation lead to increased carbon emissions, depletion of soil nutrients, and reduced carbon sequestration capacity. These activities disrupt the natural flow of critical elements like carbon, nitrogen, and phosphorus, contributing to climate change and reducing ecosystem resilience.
Acid Rain and Environmental Consequences
Acid rain is a major environmental issue that results from the release of certain pollutants into the atmosphere, which then interact with water vapor to form acidic compounds. These acids, once precipitated, can have devastating effects on ecosystems, soil quality, and even man-made structures. Human activities, such as industrial emissions and vehicle exhaust, are significant contributors to this problem.
When acid rain falls on natural habitats, it disrupts the balance of pH levels in soil and water bodies. This disturbance harms plant life, aquatic species, and soil organisms. The effects are often far-reaching, affecting the health of ecosystems and the organisms that depend on them.
Impact on Plant Life
Acid rain directly damages plant tissues and reduces soil fertility, making it harder for plants to absorb essential nutrients. Some of the key effects include:
- Soil Degradation: The acidic compounds leach essential minerals like calcium and magnesium from the soil, decreasing its fertility.
- Leaf Damage: Acid rain can cause leaves to become discolored, weakened, and more susceptible to disease.
- Reduced Growth: The overall health and growth rate of plants are significantly reduced in areas affected by acid precipitation.
Effects on Aquatic Ecosystems
Acid rain also has a profound impact on aquatic ecosystems, as it lowers the pH of lakes, rivers, and streams. This can lead to:
- Fish Mortality: A drop in pH levels can be lethal to many fish species, especially those that require a neutral pH to survive.
- Disruption of Reproduction: Acidic water affects the reproductive cycles of many aquatic organisms, decreasing their populations.
- Loss of Biodiversity: The alteration in pH levels can lead to a loss of species diversity in aquatic habitats, as only those adapted to acidic conditions survive.
In addition to these effects, acid rain also accelerates the corrosion of buildings and infrastructure, further contributing to the environmental and economic impacts of this phenomenon. Reducing emissions from industrial sources and promoting cleaner energy alternatives are essential steps in mitigating the effects of acid rain.
Environmental Chemistry and Climate Change
Human activities have significantly altered the natural processes that regulate temperature and atmospheric composition. By releasing pollutants and greenhouse gases into the air, we have enhanced the natural greenhouse effect, leading to global warming and climate instability. Understanding how these chemicals interact within the atmosphere is crucial for addressing the challenges of climate change.
Greenhouse Gas Emissions and Global Warming
One of the primary drivers of climate change is the increase in greenhouse gas emissions. Carbon dioxide, methane, and nitrous oxide trap heat in the atmosphere, causing a rise in global temperatures. These gases, primarily released through fossil fuel combustion, deforestation, and agricultural practices, have led to significant changes in weather patterns and global temperatures.
As temperatures continue to rise, various feedback loops are triggered, such as the melting of ice caps, which further amplify warming. These effects disrupt ecosystems, cause sea level rise, and increase the frequency and intensity of extreme weather events.
Impact on Ecosystems and Biodiversity
Climate change induced by human activities is having a profound effect on biodiversity. Changes in temperature, precipitation, and seasonality alter habitats, forcing species to adapt, migrate, or face extinction. The shift in ecosystems can also disrupt food chains, impacting both plant and animal populations.
Coral reefs, polar ecosystems, and tropical forests are among the most vulnerable to temperature changes. These habitats are highly sensitive to slight variations in temperature, and their disruption can lead to the loss of countless species that depend on them for survival.
To mitigate these effects, international cooperation and a shift toward sustainable practices are critical in reducing emissions and protecting vulnerable ecosystems from further degradation.
Renewable Resources and Chemical Processes
Renewable resources offer a sustainable alternative to fossil fuels, providing energy solutions that are replenished naturally over time. These resources, such as sunlight, wind, and biomass, play a crucial role in reducing dependence on non-renewable energy sources. However, their conversion into usable forms of energy involves a range of complex chemical processes that ensure efficiency and minimize environmental impact.
Conversion of Solar and Wind Energy
Solar and wind power rely on the conversion of natural energy into electricity through various chemical and physical processes. In solar energy systems, photovoltaic cells convert sunlight directly into electrical energy, relying on semiconductor materials to facilitate electron movement. Similarly, wind energy harnesses the motion of air through turbines, which convert kinetic energy into mechanical power. Both methods produce electricity with minimal chemical waste, making them environmentally friendly alternatives to traditional energy sources.
Biomass and Biofuels Production
Biomass, derived from organic materials such as plants, agricultural waste, and algae, can be converted into biofuels like ethanol and biodiesel. The conversion process often involves complex chemical reactions, such as fermentation or transesterification, where enzymes or catalysts help break down organic matter into usable fuels. These biofuels can serve as a cleaner alternative to gasoline and diesel, reducing carbon emissions and promoting sustainable energy use.
While renewable energy sources significantly reduce environmental impact, continued advancements in technology and chemical processes are necessary to improve their efficiency, scalability, and economic feasibility. Investing in research and development will be key to accelerating the transition to a more sustainable energy future.
Water Pollution and Chemical Contaminants
Water pollution is a critical issue that arises when harmful substances enter water bodies, compromising water quality and threatening aquatic life. These contaminants can come from a variety of sources, such as industrial discharges, agricultural runoff, and untreated sewage. Once in water systems, these chemicals can persist for long periods, affecting ecosystems, human health, and the availability of clean water for various purposes.
Types of Chemical Pollutants
Chemical contaminants can be categorized into several types based on their source and impact. Common pollutants include:
- Heavy Metals: Substances like mercury, lead, and arsenic are toxic and can accumulate in aquatic organisms, posing risks to wildlife and humans.
- Pesticides and Fertilizers: Runoff from agricultural lands often carries these chemicals into nearby water sources, leading to nutrient pollution and harm to aquatic life.
- Industrial Waste: Factories and manufacturing plants discharge various chemicals, such as solvents and acids, into water systems, which can contaminate both freshwater and marine environments.
Effects on Aquatic Ecosystems and Human Health
The presence of toxic chemicals in water can disrupt aquatic ecosystems by affecting the health and reproduction of fish, plants, and other organisms. For example, heavy metals can cause bioaccumulation, where toxic substances build up in the tissues of organisms over time. This can lead to health problems and even death for species within the ecosystem.
For humans, consuming contaminated water or eating contaminated fish can lead to serious health problems, such as poisoning, neurological damage, and reproductive issues. Ensuring access to clean, safe water is crucial for public health, requiring strict regulation and monitoring of water quality.
Efforts to mitigate water pollution focus on reducing chemical runoff, improving wastewater treatment, and enforcing environmental protection laws to safeguard water sources from harmful contaminants.
Soil Chemistry and Sustainable Agriculture
Soil plays a pivotal role in agricultural productivity, influencing plant growth and nutrient availability. Understanding the chemical composition and properties of soil is essential for managing land sustainably and ensuring long-term crop yields. With the growing demand for food production, it is crucial to adopt practices that maintain soil health while minimizing harmful impacts on ecosystems.
Key Soil Components and Nutrient Cycling
Soil consists of various minerals, organic matter, water, and air, each contributing to its overall health and fertility. Some of the primary components include:
- Soil pH: A measure of acidity or alkalinity, which affects nutrient availability and microbial activity in the soil.
- Macronutrients: Essential elements such as nitrogen, phosphorus, and potassium that plants require in large amounts for growth.
- Micronutrients: Trace elements like zinc, copper, and manganese, which are needed in smaller quantities but are still vital for plant development.
Healthy soil enables efficient nutrient cycling, where organic matter breaks down, releasing nutrients back into the system for future plant use. A balanced nutrient cycle is crucial for sustainable farming practices, as it reduces the need for synthetic fertilizers and supports long-term soil fertility.
Sustainable Agricultural Practices
Adopting sustainable farming techniques that focus on soil conservation is key to preserving its health and preventing degradation. Some effective methods include:
- Crop Rotation: Alternating different crops in the same field to maintain soil fertility and reduce the buildup of pests and diseases.
- Reduced Tillage: Minimizing soil disturbance to protect soil structure, reduce erosion, and preserve organic matter.
- Organic Fertilizers: Using compost, manure, or cover crops to naturally enrich soil without relying on synthetic chemicals.
These practices help restore and maintain soil health, ensuring that agricultural systems remain productive while minimizing environmental harm. By focusing on sustainable methods, we can secure food production while protecting soil for future generations.
Air Quality and Chemical Analysis
Air quality is a critical factor in maintaining public health and supporting ecosystems. The presence of pollutants in the atmosphere can have profound effects on human well-being and the natural world. Identifying and understanding the chemical composition of airborne substances is essential for controlling and mitigating pollution, as well as for implementing effective health protection measures.
Common Airborne Pollutants
Various harmful chemicals can be found in the air, originating from both natural and human-made sources. Some of the most common pollutants include:
- Particulate Matter (PM): Tiny solid or liquid particles, such as dust, soot, and smoke, which can cause respiratory issues when inhaled.
- Ozone (O3): A gas that forms when sunlight reacts with pollutants, ozone at ground level can irritate the lungs and contribute to smog formation.
- Nitrogen Oxides (NOx): Emissions from vehicles and industrial processes that contribute to acid rain and respiratory problems.
- Carbon Monoxide (CO): A colorless, odorless gas that can interfere with oxygen transport in the body, often emitted by vehicle exhaust and industrial processes.
- Sulfur Dioxide (SO2): A compound that can contribute to acid rain, originating primarily from burning fossil fuels like coal.
Methods of Chemical Analysis

Accurately measuring pollutants in the air is essential for assessing air quality and implementing regulatory measures. Various chemical analysis techniques are employed, including:
- Gravimetric Analysis: This method involves collecting particulate matter on filters and weighing the mass to determine concentration levels.
- Gas Chromatography: A technique used to separate and analyze gases in air samples, allowing for the identification of specific chemical compounds.
- Spectroscopic Techniques: Methods like UV-Visible and Infrared spectroscopy are used to measure the presence of various gases and particulate matter by analyzing light absorption.
Understanding the concentration and behavior of these chemicals in the atmosphere allows scientists to track pollution levels and predict potential health impacts, making chemical analysis an essential tool for improving air quality management.
The Ozone Layer and Chemical Reactions
The protective layer high in Earth’s atmosphere plays a crucial role in shielding life from harmful ultraviolet radiation. This layer is composed of a specific form of oxygen and its balance is essential for maintaining a safe living environment. Various natural and human-driven chemical processes directly influence the stability of this layer, either by strengthening or depleting its concentration. Understanding the reactions that occur within this layer is vital for evaluating its ongoing health and the impacts on the planet.
Key Reactions Involved in Ozone Formation

Ozone forms when oxygen molecules are broken apart by ultraviolet light, leading to the creation of highly reactive oxygen atoms. These atoms then interact with other oxygen molecules, leading to the formation of ozone. The overall process can be summarized in the following reactions:
- Photodissociation of Oxygen (O2): Ultraviolet light breaks down oxygen molecules into individual oxygen atoms (O + O2 → O3).
- Ozone Creation: Free oxygen atoms (O) combine with existing oxygen molecules (O2) to form ozone (O3) through a chemical reaction.
Destruction of Ozone and Contributing Factors
While ozone is constantly being created and destroyed in the stratosphere, certain chemicals, particularly human-made compounds, disrupt this balance. Chlorofluorocarbons (CFCs) are one of the primary agents responsible for ozone depletion. These chemicals, once released into the atmosphere, break down ozone molecules, thinning the protective layer. The chemical reactions involved in ozone destruction include:
- Breakdown of CFCs: CFCs released into the atmosphere rise and, under UV light, release chlorine atoms (Cl) that catalyze the breakdown of ozone.
- Ozone Depletion: Chlorine atoms react with ozone molecules, causing them to break apart into oxygen molecules and single oxygen atoms (Cl + O3 → ClO + O2).
These reactions accelerate the depletion of the ozone layer, leading to the thinning of this crucial shield and increasing exposure to harmful UV radiation, which can lead to serious health and ecological consequences.
Waste Management and Chemical Solutions
Effective waste disposal plays a critical role in maintaining public health and reducing environmental harm. Various methods, often involving chemical treatments, are utilized to break down or neutralize hazardous substances found in waste. By employing innovative chemical processes, waste can be converted into less harmful materials, reducing its impact on ecosystems and human populations. This section explores how chemicals are used to manage and treat different types of waste, providing essential solutions for a cleaner and safer future.
Chemical Methods in Waste Treatment
Chemical treatments are crucial for handling various forms of waste, especially hazardous ones like industrial byproducts, medical waste, and municipal solid waste. These methods aim to neutralize toxic substances, reduce volume, or transform waste into reusable materials. Some common chemical solutions include:
| Waste Type | Chemical Process | Outcome |
|---|---|---|
| Industrial Waste | Neutralization with acids or bases | Conversion into non-toxic substances |
| Medical Waste | Autoclaving or chemical sterilization | Destruction of pathogens |
| Municipal Solid Waste | Incineration with chemical catalysts | Reduction of waste volume, energy generation |
Challenges and Advances in Chemical Waste Solutions
Despite significant progress in chemical waste treatment technologies, challenges remain in fully addressing the harmful effects of waste. New advances focus on enhancing the efficiency of chemical processes, minimizing harmful emissions, and finding sustainable solutions for recycling and waste reduction. Key developments include:
- Advanced Oxidation Processes (AOPs): These methods use powerful oxidants like ozone or hydrogen peroxide to break down complex pollutants.
- Bioremediation: The use of microorganisms or enzymes to degrade organic waste, often with the assistance of chemical additives.
- Recycling of Chemical Waste: Processes that recover valuable materials from waste, reducing the need for raw resources.
By continuously improving and adopting innovative chemical solutions, waste management practices can become more sustainable, reducing environmental impact and promoting a circular economy.
Industrial Chemistry and Environmental Concerns
Industrial processes have significantly shaped modern society, providing essential products and energy. However, these activities often result in negative impacts on ecosystems and public health. From emissions of harmful gases to the disposal of toxic waste, industries contribute to a variety of challenges that require innovative solutions. Understanding the relationship between industrial production and its effects on natural systems is crucial for developing sustainable practices that minimize harm while continuing to support economic growth.
Key Issues Linked to Industrial Practices
Industries, particularly manufacturing and chemical production, release various pollutants that affect air, water, and soil quality. Some of the major concerns include:
- Air Pollution: Factories emit large quantities of carbon dioxide, sulfur compounds, and particulate matter that contribute to smog, acid rain, and global warming.
- Water Contamination: Industrial waste, often containing heavy metals, toxins, and chemicals, can leak into rivers and groundwater, harming aquatic life and human health.
- Soil Degradation: Improper disposal of industrial byproducts, including hazardous chemicals, can lead to long-term soil pollution, reducing land fertility and biodiversity.
Innovations and Solutions for Reducing Impact

In recent years, industries have begun adopting more sustainable practices, focusing on reducing waste and improving resource efficiency. Some of the methods being employed include:
- Green Manufacturing: This approach incorporates energy-efficient technologies and renewable materials to reduce resource consumption and minimize harmful emissions.
- Waste Minimization: Techniques such as waste-to-energy and recycling of byproducts are used to divert waste from landfills and reduce environmental pollution.
- Carbon Capture and Storage: Technologies that capture carbon dioxide emissions from industrial facilities and store them underground help mitigate the effects of global warming.
While industrial practices will continue to be a vital part of the global economy, addressing the environmental concerns they pose is essential for ensuring a sustainable future. Continued innovation and adherence to stricter regulations are necessary to balance production with preservation of natural resources.
The Role of Chemists in Environmental Protection
Professionals in science play a critical role in addressing ecological challenges and developing solutions that protect natural resources. Through research and innovation, experts focus on identifying and mitigating harmful substances, reducing pollution, and creating sustainable materials. By understanding molecular interactions and designing effective systems, these individuals contribute to preserving ecosystems and improving public health.
Innovative Solutions for Pollution Control
One of the key contributions made by these specialists is the development of new methods to control and reduce pollution. They design processes that neutralize or eliminate toxins released into air, water, and soil. For example, creating biodegradable alternatives to harmful plastics, or inventing filters that can remove heavy metals from contaminated water, plays a major role in minimizing harmful substances in nature.
Designing Green Technologies
Another significant aspect of their work is in designing and promoting “green” technologies. These systems rely on renewable resources and minimize energy consumption. For instance, chemists are behind the development of solar panels, biofuels, and hydrogen-powered devices that reduce reliance on fossil fuels. These innovations are essential for transitioning towards a more sustainable and eco-friendly energy landscape.
Monitoring and Assessing Ecological Impact
In addition to creating new technologies, experts continuously monitor existing processes to evaluate their impact on ecosystems. Through chemical analysis, they track pollutants in various ecosystems, allowing for early detection of potential hazards. By establishing better guidelines and standards, they help industries comply with regulations that protect both human health and wildlife.
In essence, these experts provide valuable insight and technology to help society make more informed decisions that balance progress with conservation. Through their work, industries and governments are better equipped to address complex environmental issues and work toward a healthier planet.
Methods for Reducing Environmental Impact
Numerous strategies are available to reduce the harmful effects of human activities on natural systems. These approaches focus on minimizing resource consumption, reducing waste production, and promoting sustainability across various industries. By embracing these practices, societies can make significant strides toward preserving ecosystems, reducing pollution, and fostering long-term ecological balance.
Energy Efficiency and Transition to Renewable Sources
Improving energy use and shifting toward cleaner energy sources are essential steps in reducing harmful emissions. Energy-efficient technologies can significantly lower the amount of energy consumed, while renewable energy options offer a sustainable alternative to fossil fuels. Key strategies include:
- Implementing energy-saving technologies in households and industries, such as LED lighting and energy-efficient appliances.
- Expanding the use of renewable energy sources like wind, solar, and geothermal power.
- Promoting public transportation and electric vehicles to reduce fuel consumption and air pollution.
Waste Management and Recycling
Efficient waste management practices, including recycling and composting, are crucial in minimizing pollution. By reusing materials and reducing the amount of waste that ends up in landfills, societies can decrease the impact on land and water resources. Some methods for effective waste reduction include:
- Implementing comprehensive recycling programs that include paper, plastic, and metal collection.
- Encouraging composting of organic waste to reduce methane emissions and enhance soil health.
- Promoting the use of biodegradable materials in packaging and products.
Conservation of Resources and Ecosystems
Protecting natural resources and ecosystems is critical for maintaining biodiversity and ensuring the availability of resources for future generations. Sustainable practices in agriculture, forestry, and water management are key to reducing human impact on natural habitats. Some strategies for resource conservation include:
- Adopting sustainable agricultural practices, such as crop rotation and organic farming, to preserve soil quality.
- Using water-efficient irrigation systems to reduce water waste in agriculture.
- Protecting and restoring natural habitats to maintain biodiversity and ecosystem services.
Technological Solutions for a Sustainable Future

Innovative technologies play a vital role in minimizing environmental degradation. From carbon capture systems to smart grids, technological advancements help reduce emissions and optimize resource use. Below are a few examples of impactful technological solutions:
| Innovation | Impact |
|---|---|
| Carbon Capture and Storage (CCS) | A technique for capturing carbon dioxide emissions from industrial sources and storing them underground, preventing them from reaching the atmosphere. |
| Electric Vehicles (EVs) | Vehicles powered by electricity that eliminate the need for fossil fuels and reduce air pollution. |
| Smart Grids | Advanced electrical grids that optimize energy distribution and enhance efficiency, reducing waste and lowering carbon footprints. |
In conclusion, reducing ecological impact requires a multifaceted approach, including adopting cleaner technologies, conserving resources, and improving waste management. These efforts not only benefit nature but also support a sustainable future for generations to come.
Future Directions in Environmental Chemistry
As global challenges related to sustainability, pollution, and resource management continue to evolve, innovative approaches to addressing these issues are becoming more critical. Future progress in this field will depend on advancements in sustainable practices, novel technologies, and a deeper understanding of natural systems. By developing new methods to reduce waste, lower emissions, and manage resources more effectively, it is possible to create a more harmonious relationship between human activities and ecosystems.
Emerging Technologies for Pollution Control
One of the key areas of focus will be the development of new technologies to mitigate pollution. As industries grow and urban areas expand, more advanced solutions will be required to manage the increasing environmental load. Some promising developments include:
- Advanced filtration and purification systems to remove harmful substances from air and water.
- Biodegradable materials that can replace conventional plastics, significantly reducing long-term pollution.
- Green solvents and catalysts for industrial processes that minimize harmful emissions and waste.
Advancing Sustainable Resource Management
Another priority for the future will be improving how resources are managed and utilized. Sustainable agriculture, renewable energy, and circular economies are all areas in need of continued innovation. Some future directions include:
- Development of closed-loop systems that recycle materials back into production processes, reducing waste.
- Efficient use of renewable energy sources, such as solar, wind, and hydroelectric, to replace fossil fuels.
- Improved agricultural practices that reduce water consumption, minimize soil degradation, and promote biodiversity.
By embracing these strategies and focusing on new solutions, it is possible to create a cleaner, more sustainable future. Research and innovation in this area will be essential to overcoming current and future environmental challenges.