Mystery Powders Lab Answers and Solutions
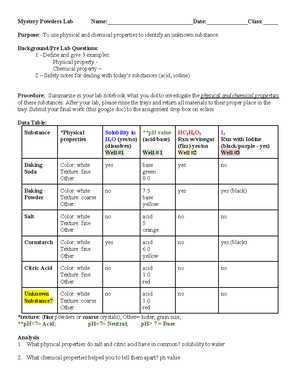
In scientific experiments, the identification of unfamiliar materials can be both intriguing and challenging. By applying various techniques, one can uncover the properties that define each substance. This process involves systematic observations, chemical reactions, and physical tests that reveal key characteristics, allowing for accurate identification.
Understanding how to perform these tests and interpret their outcomes is crucial for anyone conducting similar analyses. It requires a combination of knowledge, careful observation, and sometimes a bit of trial and error. Whether testing for solubility, acidity, or reactivity, each test provides valuable insights into the composition and behavior of the substance in question.
Through this approach, complex mixtures or unfamiliar materials can be sorted and understood. By the end of the process, the once unknown becomes familiar, offering both a sense of accomplishment and a deeper understanding of the principles at play in the world of chemistry.
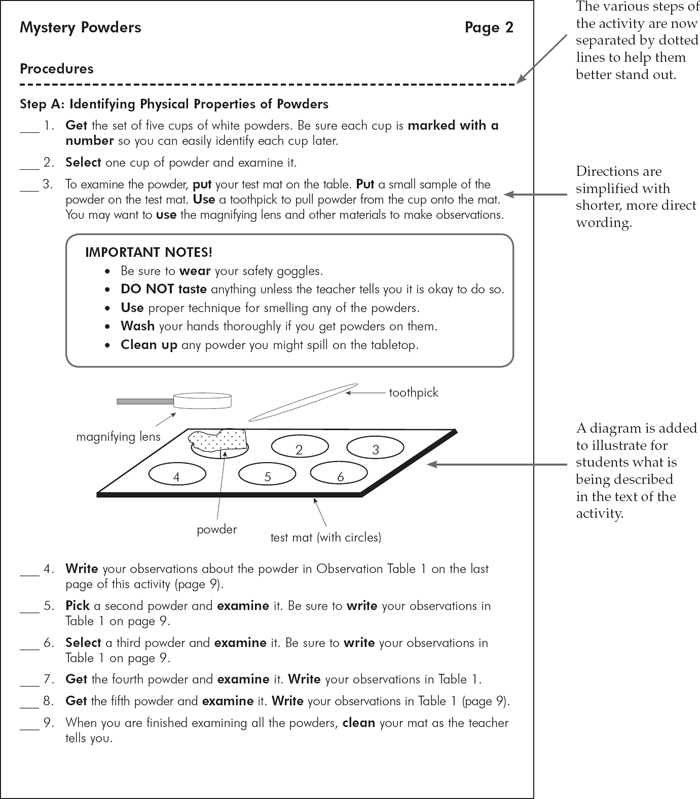
Mystery Powders Lab Answers Overview
The process of identifying unknown substances involves a series of systematic tests and observations. These experiments are designed to reveal the unique properties of each material, such as its solubility, acidity, and reactivity to various chemicals. The goal is to distinguish one substance from another by carefully analyzing their behavior in different conditions.
Throughout the experiment, several methods are employed to gather data, including physical tests like dissolving the material in water, heating it, or observing color changes. Chemical reactions play a key role in determining the identity of the substance, as different compounds react in predictable ways with specific reagents. These reactions provide critical clues about the composition of the material.
By the end of the process, the previously unknown substance is categorized based on its distinct characteristics. The ability to recognize and interpret these properties allows scientists and students alike to draw meaningful conclusions and deepen their understanding of chemical principles.
Understanding the Mystery Powders Experiment
The process of analyzing unfamiliar materials through controlled testing is both an educational and practical exercise in chemistry. In this type of experiment, participants explore a variety of unknown substances and apply different testing methods to determine their identity. By observing their reactions and properties under various conditions, it becomes possible to deduce their characteristics and classification.
The experiment typically begins with a set of unknown materials, each requiring specific tests to reveal its unique properties. These properties are examined through simple procedures, such as checking solubility in water, testing for pH levels, or observing any changes when heat or chemical reagents are applied. The goal is to systematically narrow down the possibilities and confidently identify the substance.
Below is a table summarizing the common tests used to analyze these substances:
| Test | Purpose | Expected Result |
|---|---|---|
| Solubility Test | To check if the substance dissolves in water | Soluble materials dissolve in water; insoluble materials do not |
| Acidity Test | To determine if the substance is acidic, neutral, or alkaline | Acidic substances turn litmus paper red, alkaline turn it blue |
| Heat Test | To observe changes when the substance is heated | Some substances melt, burn, or change color when heated |
| Reaction with Chemicals | To identify specific chemical reactions | Different substances react with specific reagents, such as bubbling or color change |
By performing these simple yet effective tests, each unknown substance is gradually analyzed, leading to a clearer understanding of its nature and classification. Through this experiment, students and scientists gain practical experience in using scientific methods to identify and characterize materials.
How to Identify Unknown Powders

Identifying unknown substances requires a systematic approach that combines observation, experimentation, and chemical analysis. Each material exhibits unique characteristics, and by performing specific tests, it is possible to uncover its identity. Through careful procedures, one can gather clues to differentiate substances and categorize them based on their behavior under certain conditions.
Step 1: Physical Properties Examination

Begin by observing the physical characteristics of the unknown material. This includes texture, color, and any visible markings or odors. Solubility in water can also be an initial test, as some substances dissolve while others remain intact. Physical properties often provide the first insights into the nature of the material, though further testing is usually required to confirm its identity.
Step 2: Chemical Reaction Tests

Chemical testing involves applying reagents or exposing the material to heat to observe how it reacts. For example, some substances may change color, emit gases, or produce other distinct reactions when combined with certain chemicals. These reactions are a valuable tool for narrowing down the possibilities and confirming the composition of the unknown material.
By combining these methods, you can systematically eliminate options and identify the substance with greater confidence. Through careful observation and testing, unknown materials are revealed, providing both educational and practical insights into their composition.
Key Chemical Reactions in the Lab
Chemical reactions are fundamental to identifying unknown substances and understanding their properties. By observing how materials interact with reagents or other elements, scientists can draw important conclusions about their composition. These reactions can manifest in various forms, such as color changes, the release of gases, or the formation of precipitates, each providing valuable clues about the identity of the material under investigation.
Common Reactions and Their Significance

Certain reactions are commonly used to identify materials in a controlled environment. For example, reactions that produce gas bubbles can indicate the presence of acidic or alkaline compounds. Other reactions, such as the formation of a precipitate, may suggest the presence of certain metal ions or salts. Understanding these reactions is essential for distinguishing one substance from another.
Testing for Specific Chemical Properties
The following table outlines some common chemical reactions used to test for specific properties in substances:
| Reaction | Purpose | Expected Outcome |
|---|---|---|
| Reaction with Hydrochloric Acid | To test for carbonates or metals | Bubbling or effervescence indicating the presence of carbonates or metal reactions |
| Reaction with Silver Nitrate | To test for halides (e.g., chloride, bromide) | Formation of a white or cream precipitate indicating halides |
| Reaction with Sodium Hydroxide | To test for metal ions | Color change or precipitate formation depending on the metal ion present |
| Flame Test | To identify metal salts | Characteristic flame colors indicating specific metal ions |
By understanding these key reactions and their outcomes, it becomes easier to narrow down the possibilities and confidently identify substances in the experiment. Each reaction serves as a tool in a larger puzzle, helping to piece together the characteristics of the unknown material.
Testing for Acidity and Alkalinity
Determining whether a substance is acidic, neutral, or alkaline is essential in identifying its chemical properties. The pH level of a material influences its behavior in reactions and its interactions with other substances. By testing the acidity or alkalinity, we gain insight into its molecular structure and predict how it will respond under different conditions.
One of the most common methods for testing pH involves using litmus paper or universal indicator solutions. These tools change color depending on the pH level, providing a quick and simple way to categorize the substance. Acidic materials will typically turn the paper red, while alkaline substances will shift the color to blue. A neutral substance will show little or no color change.
Additional techniques, such as using pH meters, offer a more precise measurement of acidity or alkalinity. This method is often used when exact values are necessary for further analysis or when working with more complex substances. By accurately identifying the pH, scientists can predict how the substance might react in different environments or when mixed with other chemicals.
Identifying Powders Using Solubility

Solubility is one of the key tests for identifying an unknown substance. By observing how a material dissolves in water or other solvents, scientists can determine important characteristics about its composition. Some substances dissolve easily, while others remain unchanged, providing clues to their chemical nature and helping to narrow down potential identities.
Solubility Testing Methods
The simplest way to test solubility is to add the substance to a solvent such as water and observe what happens. If the substance dissolves completely, it is considered soluble; if it does not, it is insoluble. However, solubility can also depend on temperature, as some materials dissolve better when heated.
Common Solubility Results
The table below outlines how different types of substances typically behave in a solubility test:
| Substance | Solubility in Water | Characteristic Behavior |
|---|---|---|
| Salt (Sodium Chloride) | Highly soluble | Forms a clear solution when dissolved |
| Sugar | Highly soluble | Forms a sweet solution when dissolved |
| Sand | Insoluble | Remains solid, does not dissolve |
| Oil | Insoluble | Forms separate layers, does not dissolve in water |
By performing a solubility test, one can quickly categorize substances as either soluble or insoluble, which helps to further isolate their possible identities. While solubility alone may not provide a definitive answer, it is an important step in the identification process, often leading to additional tests and observations.
Using Heat to Analyze Powders
Applying heat to a substance is a powerful method for understanding its chemical and physical properties. When exposed to heat, materials can undergo changes such as melting, decomposition, or the release of gases. These reactions provide valuable clues about the composition of the substance and help distinguish one material from another.
Heating can reveal several key characteristics. For example, some compounds may melt at certain temperatures, while others may burn, change color, or even react with the atmosphere. These reactions often indicate the presence of specific elements or compounds and can be used to narrow down the list of potential identities.
In some cases, the way a substance reacts to heat can help identify impurities or confirm its purity. For instance, if a substance does not melt or change under heat, it may suggest that it is a stable, pure material. On the other hand, a strong reaction could indicate a mixture of compounds or a substance that is reactive at high temperatures.
Observing Physical Properties of Powders
Examining the physical characteristics of a material is a crucial first step in identifying it. By carefully observing its appearance, texture, and behavior under various conditions, one can gather valuable clues about the substance’s identity. These properties often provide initial insights into the material’s composition and potential uses.
Key Physical Properties to Observe

The following physical properties are commonly observed when analyzing an unknown substance:
- Color: The color of a substance can indicate the presence of certain chemical elements or compounds.
- Texture: Whether a material is gritty, smooth, or powdery can give hints about its structure and composition.
- Density: The density of a substance, often determined by its mass-to-volume ratio, can help differentiate between materials with similar appearances.
Behavior Under Different Conditions
In addition to the visual inspection, observing how a material reacts when exposed to light, temperature changes, or different solvents can offer more clues. For example, some substances may clump together when moistened, while others may remain dispersed. Such reactions often reveal further details about their molecular structure and possible chemical properties.
Interpreting Lab Results Effectively
Interpreting experimental data accurately is essential for drawing meaningful conclusions. The results obtained during experiments often require careful analysis to ensure that they align with expectations and to determine what they reveal about the substances under investigation. A thorough understanding of the procedures and observations helps to connect experimental outcomes with theoretical knowledge.
Effective interpretation involves considering all variables that could have influenced the results. For example, changes in temperature, the presence of impurities, or even the timing of certain reactions can all affect the outcome. A comprehensive approach considers these factors, ensuring that the conclusions drawn are valid and reliable.
Cross-referencing results with known information or previous studies can further confirm findings. This comparative analysis helps to eliminate uncertainties and strengthen the interpretation. Additionally, using control experiments or repeating tests can provide more consistency and reliability, increasing confidence in the conclusions.
Critical thinking is key when interpreting results, as it allows one to assess the implications of each observation, weigh the evidence, and make informed decisions about the next steps in the investigative process. By following a structured approach and remaining objective, scientists can interpret their findings effectively and accurately.
Common Powders in Mystery Experiments
In many experiments designed to identify unknown substances, certain materials are frequently encountered due to their distinct physical and chemical properties. Understanding the characteristics of these common substances can help narrow down the potential identities of unknown samples. These substances are often chosen for their well-documented behaviors under different test conditions, which makes them ideal candidates for identification tests.
Among the most common substances used in such experiments are salts, acids, and organic compounds. Each of these materials reacts differently with solvents, heat, and chemical indicators, allowing for easy differentiation. For example, a crystalline substance that dissolves easily in water may indicate the presence of a salt, while a white powder that fizzes in the presence of an acid could suggest an alkaline material.
Salts, such as sodium chloride, are frequently encountered due to their solubility and stability. These substances are often used to test for dissolving properties and to observe changes in conductivity. Acids, like citric or tartaric acid, are commonly used to test reactivity with bases or their behavior when mixed with metals. Organic compounds, such as starch or sugar, offer insight into the material’s biological origin and can be identified through reactions with iodine or other reagents.
By recognizing the characteristics of these substances and understanding how they react under various conditions, it becomes easier to identify unknown materials in experimental settings.
Safety Precautions in the Lab

When conducting experiments involving unknown substances, ensuring safety is the top priority. Handling chemicals and other materials requires careful attention to avoid accidents, contamination, or exposure to harmful reactions. Adhering to proper safety protocols is essential for the well-being of everyone in the experimental environment.
There are several key safety measures that should be followed at all times:
- Wear protective gear: Always wear safety goggles, gloves, and lab coats to protect against spills, splashes, and accidental exposure to harmful substances.
- Use proper ventilation: Ensure that the workspace is well-ventilated, especially when working with chemicals that produce fumes or vapors.
- Know emergency procedures: Be familiar with the location and use of emergency equipment such as fire extinguishers, eyewash stations, and first aid kits.
- Handle materials with care: Always handle chemicals and substances according to the guidelines, and avoid direct contact unless necessary for the experiment.
Additionally, certain precautions should be taken when working with heat or electrical equipment:
- Monitor heat sources: Never leave heat sources unattended, and always ensure they are turned off when no longer needed.
- Check equipment before use: Inspect electrical equipment and connections to ensure they are in good working condition to prevent electrical hazards.
By following these safety measures, the risks associated with handling unfamiliar materials can be minimized, creating a safer environment for conducting experiments and obtaining accurate results.
Using Indicators to Test Substances
Indicators are substances that change color or exhibit other visible changes in response to the presence of specific chemicals. These changes can provide valuable insights into the chemical properties of an unknown material. By using various indicators, one can quickly assess whether a substance is acidic, basic, or neutral, or if it contains certain ions or compounds.
Commonly used indicators include:
- Litmus paper: This simple tool is used to test for acidity or alkalinity. Blue litmus paper turns red in acidic conditions, while red litmus paper turns blue in basic environments.
- Phenolphthalein: This indicator is colorless in acidic solutions but turns pink as the solution becomes more basic.
- Universal indicator: A mixture of several indicators, this solution provides a color gradient from red (strongly acidic) to purple (strongly alkaline), making it useful for determining the pH of a solution.
- Silver nitrate: This solution is used to detect the presence of chloride ions, forming a white precipitate when chloride is present.
By carefully choosing and using the appropriate indicator, one can obtain quick and reliable results to help identify the chemical nature of a substance. These tests can be easily performed with minimal equipment, making them ideal for initial analysis in many experiments.
Powder Identification Using Reagents
Reagents are chemical substances that react with certain compounds, allowing scientists to identify unknown materials based on their reactivity. By adding specific reagents to a sample, it is possible to observe characteristic changes, such as color shifts, precipitate formation, or gas release, which can provide critical clues about the identity of the material being tested.
Common reagents used for identifying various substances include:
- Barium chloride: Used to test for sulfate ions, forming a white precipitate if sulfates are present.
- Hydrochloric acid: A common reagent to check for the presence of carbonates; it produces carbon dioxide gas when reacting with carbonates.
- Ammonia solution: This reagent can be used to identify metals such as copper and iron, as it forms colored complexes with certain metal ions.
- Iodine solution: This reagent is used to test for starch presence, turning blue-black when starch is present.
- Potassium permanganate: A strong oxidizing agent used to test for the presence of reducing agents; it typically decolorizes when it reacts with certain compounds.
By observing the reactions between reagents and unknown materials, it becomes possible to identify specific chemical components, guiding further analysis and helping to confirm or exclude potential identities of the substances. This approach is widely used in both educational and professional settings for its simplicity and reliability.
Step-by-Step Guide to Lab Procedures

Conducting experiments with unknown substances requires a methodical approach to ensure accurate results. Following clear, systematic procedures helps minimize errors and allows for proper identification based on chemical and physical reactions. The following steps outline a typical approach to analyzing substances, ensuring safety and consistency throughout the process.
Preparation and Safety
- Wear protective gear: Always wear gloves, goggles, and a lab coat to protect against chemical exposure.
- Set up your workspace: Ensure that all necessary equipment, such as test tubes, beakers, and reagents, are within easy reach and in good condition.
- Ensure ventilation: If working with volatile or hazardous substances, perform the experiment in a well-ventilated area or under a fume hood.
Testing and Observations
- Initial observation: Begin by noting the physical characteristics of the substance, including color, texture, and odor (if safe to do so).
- Conduct basic tests: Test the solubility, pH, and reactivity with common reagents to gather preliminary information about the substance.
- Record all observations: Keep detailed notes of each test result, including any changes in color, temperature, or the formation of solids or gases.
By following these steps, you can systematically analyze an unknown material, ensuring that all key properties are assessed and recorded. This approach helps in reaching conclusions about the composition and identity of the substance under investigation.
Practical Applications of Powder Analysis
Understanding the composition of various substances is critical in numerous industries, from pharmaceuticals to environmental science. By analyzing unfamiliar materials, we can ensure their safety, effectiveness, and suitability for specific applications. Whether identifying contaminants in food, determining the purity of a chemical, or testing materials for construction, such analyses provide valuable insights into the properties and uses of these substances.
Pharmaceutical Industry
In the pharmaceutical field, analyzing substances is vital for ensuring the purity and efficacy of drugs. By identifying the chemical components of powders used in medication, manufacturers can guarantee that the active ingredients are present in the correct concentrations, and any contaminants are absent. This process plays a crucial role in maintaining safety standards and regulatory compliance.
Environmental and Forensic Applications
Environmental scientists often use substance analysis to identify pollutants in soil or water samples. Similarly, forensic experts analyze substances found at crime scenes to trace materials or confirm suspicions about the nature of an event. In both cases, accurate identification allows for the development of appropriate remediation or investigative strategies.
Powder analysis, therefore, extends far beyond the confines of basic chemistry and plays a crucial role in improving safety, quality control, and public health in various industries.