Chromatography Exam Questions and Answers Guide
Preparing for a test on separation techniques requires a strong grasp of various scientific methods used to analyze mixtures. These techniques are fundamental to many fields, including chemistry, biology, and environmental science. To succeed, it is crucial to understand the underlying principles, the equipment involved, and how different factors influence results.
Mastering these principles allows for efficient problem-solving and accurate interpretation of results. Whether you are dealing with liquid, gas, or solid phases, recognizing how each method works and when to apply it is essential for success. Practice with sample problems can help reinforce your knowledge and sharpen your critical thinking skills.
By familiarizing yourself with common topics and test formats, you can approach your preparation with confidence. This section will guide you through key topics, providing insight into what you should focus on, along with practical tips to enhance your readiness.
Chromatography Test Preparation Tips
To succeed in tests involving separation techniques, it is crucial to thoroughly understand both theoretical concepts and practical applications. These assessments typically focus on your ability to identify, explain, and apply various methods used in analyzing mixtures. By practicing with sample problems, you can familiarize yourself with common formats and the types of challenges you may face.
Key Areas to Focus On
Concentrate on mastering the core principles behind each method, such as the role of phases, the influence of solvents, and the mechanics of separation. Understanding the advantages and limitations of different approaches, such as liquid or gas-based techniques, will also help in tackling more complex scenarios. Reviewing practical examples and their solutions can enhance your ability to answer more intricate questions with confidence.
Common Types of Challenges
Expect to encounter problems that require you to interpret results, identify correct setups, or troubleshoot potential issues with apparatus. Being able to analyze data and draw conclusions from chromatographic output is essential. Practicing these types of problems ensures that you can handle any related queries efficiently during assessments.
Understanding the Basics of Chromatography
The foundation of any analysis technique lies in understanding the separation of components in a mixture. The process involves distributing the mixture between two phases: one that moves and one that remains stationary. This interaction results in the separation of substances based on their different affinities for each phase. Mastering this basic principle is crucial for effectively using various separation methods.
Phases Involved in Separation
The two primary phases in this method are the stationary phase and the mobile phase. The stationary phase remains fixed in place, while the mobile phase moves through it, carrying the components of the mixture along. The rate at which different substances travel depends on their interaction with both phases. Understanding this dynamic is key to predicting how substances will separate under various conditions.
Factors Influencing Separation
Several factors can affect the efficiency of the separation process, including the choice of solvent, temperature, and the nature of the materials involved. Each factor influences how substances interact with the phases, ultimately determining the speed and quality of separation. Familiarizing yourself with these variables will help in optimizing the process for specific analytical needs.
Key Concepts in Chromatographic Separation
Understanding the core principles behind separating substances in a mixture is essential for effective analysis. At the heart of this process is the differential movement of components based on their interactions with two distinct environments: one stationary and one moving. The efficiency of separation is determined by how each substance interacts with these phases, which varies depending on their chemical properties.
One important concept is the affinity of a substance for each phase. Substances with a strong attraction to the stationary phase will move more slowly, while those attracted to the mobile phase will travel faster. This difference in movement is what allows for the components to be separated and identified. The ability to control and manipulate these interactions is a key factor in achieving accurate results.
Another vital aspect is the choice of materials used in the separation process. The composition of the phases–whether solid, liquid, or gas–plays a critical role in how well the components are separated. Adjusting factors like the polarity of the phases or the type of solvent used can significantly impact the separation efficiency and resolution.
Types of Chromatography Explained
There are several methods used for separating mixtures into their individual components. Each technique utilizes different principles of interaction between the mixture and the phases involved. Understanding the distinctions between these methods is crucial for selecting the most suitable one for specific analytical tasks.
Common Separation Techniques
These methods can be broadly classified into categories based on the phase system used–whether it involves a liquid, gas, or solid phase. Some techniques rely on the movement of the mobile phase through a stationary medium, while others use a variety of solvents to achieve separation. Below is a table summarizing the most common types and their key characteristics:
| Technique | Mobile Phase | Stationary Phase | Common Applications |
|---|---|---|---|
| Thin Layer | Liquid | Solid (coated on a plate) | Identifying components in simple mixtures |
| High Performance Liquid | Liquid | Solid (packed in a column) | Complex sample analysis, pharmaceutical testing |
| Gas | Gas | Solid or liquid (coated in a column) | Environmental testing, purity analysis |
Choosing the Right Method
Each technique has its advantages depending on the nature of the sample and the required resolution. For example, gas-based methods are ideal for volatile compounds, while liquid-based approaches are better suited for analyzing larger or more complex molecules. Understanding the differences helps to select the most effective approach for achieving accurate and reproducible results.
Important Chromatographic Techniques for Assessments
When preparing for assessments involving separation processes, it is essential to focus on mastering the most commonly used methods. These techniques are integral to understanding how mixtures are separated and analyzed in various fields. Each method has unique characteristics that make it suitable for specific applications. Familiarity with these techniques will ensure you’re able to tackle related problems effectively.
The following techniques are fundamental and often tested for their ability to separate different components in a sample:
- Thin Layer Separation: A simple yet effective method for analyzing mixtures with minimal equipment.
- High Performance Liquid Separation: This approach is vital for resolving complex mixtures in the pharmaceutical and chemical industries.
- Gas-Based Methods: Used for analyzing volatile substances, this technique is crucial in environmental testing and purity analysis.
Additionally, understanding the principles behind these techniques will help you anticipate how to apply them to various types of problems:
- Identify which method is best suited for a particular sample.
- Understand how different substances interact with the stationary and mobile phases.
- Know the steps involved in performing each technique, from sample preparation to result interpretation.
Being able to explain the advantages and limitations of each approach will further strengthen your grasp on the topic. Through practice and focused study, you can develop the skills needed to tackle challenges effectively in assessments.
Sample Preparation for Chromatographic Tests
Proper preparation of samples is crucial for achieving accurate and reliable results in separation methods. The process involves ensuring that the sample is clean, concentrated, and in a suitable form for analysis. Inadequate preparation can lead to poor separation, contamination, or incorrect readings, affecting the overall outcome of the test.
Key steps in preparing a sample for testing include:
- Purification: Ensuring the sample is free from contaminants that could interfere with the separation process.
- Concentration: Adjusting the sample to a level where the components are detectable without overloading the system.
- Solubility: Dissolving the sample in the appropriate solvent to ensure that all components are in a form that can be separated efficiently.
In addition to these basic steps, understanding the specific requirements of the method being used is essential. For example, for liquid-based separations, ensuring that the sample is not too viscous and that it can flow smoothly through the system is important. Likewise, for gas-based methods, the sample should be volatile enough to vaporize without decomposing.
- Choose the correct solvent for dissolving the sample.
- Ensure the sample is filtered to remove any large particles.
- Verify the sample’s concentration is within the optimal range for detection.
By following these guidelines, you can improve the quality of your results and make the separation process more efficient, regardless of the method being used.
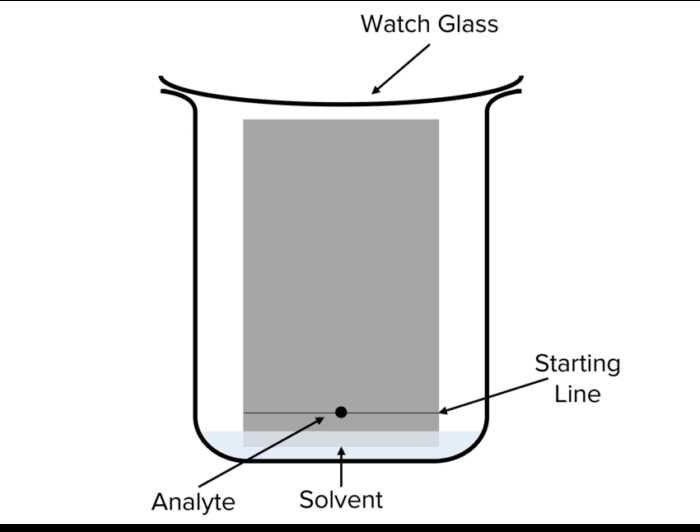
Common Questions on Thin Layer Chromatography
When studying separation techniques, thin layer methods are frequently discussed due to their simplicity and efficiency. However, several points often arise regarding the practical applications and theoretical principles of this technique. Understanding these common aspects can significantly improve your ability to apply it correctly and interpret the results effectively.
Here are some of the most frequently asked queries related to thin layer separation:
- What materials are used for the stationary phase? The stationary phase is typically a thin layer of silica gel or alumina spread on a flat plate, providing the surface for separation.
- How do you choose the right solvent? The solvent or mobile phase should be selected based on the polarity of the substances being separated. A good solvent will allow for the appropriate movement of compounds without too much overlap.
- Why is the solvent level important? The solvent level must not exceed the level of the sample spots, as this would cause the compounds to dissolve into the solvent pool, leading to inaccurate results.
- What is the significance of Rf values? The Rf value, which represents the ratio of the distance traveled by a compound to the distance traveled by the solvent, helps in identifying substances by comparing with known standards.
By addressing these common concerns, individuals can better prepare for using thin layer techniques effectively and gain a deeper understanding of how to interpret the results in various analytical settings.
High Performance Liquid Chromatography Overview
This advanced separation technique is widely used for analyzing complex mixtures, particularly in the pharmaceutical and chemical industries. By using high pressure to push the sample through a column packed with a stationary phase, this method allows for the rapid and precise separation of various components. The combination of precise control and highly sensitive detection makes it ideal for separating substances in very low concentrations.
Key components of the system include the mobile phase, the stationary phase, the column, and the detector. These elements work together to ensure the accurate separation of substances based on their differing chemical properties. Below is a table summarizing the main parts of the system:
| Component | Description |
|---|---|
| Mobile Phase | A solvent or mixture that moves through the column, carrying the sample. |
| Stationary Phase | The material packed inside the column, usually a fine silica or polymer, which interacts with the sample components. |
| Column | A cylindrical tube filled with the stationary phase through which the mobile phase flows. |
| Detector | A device that identifies and records the components as they exit the column, often using UV or fluorescence methods. |
This method is especially valuable when high precision and fast results are necessary. It has applications ranging from purity testing and quality control to environmental monitoring and forensic analysis.
Gas Chromatography: Key Points to Know
Gas-based separation techniques are essential for analyzing volatile compounds in a wide range of fields, from environmental monitoring to pharmaceutical research. This method relies on a gaseous mobile phase to carry the sample through a column, where separation occurs due to differences in the interaction between the substances and the stationary phase. The method is known for its speed, precision, and ability to handle complex mixtures.
Key factors to understand when working with this technique include the components of the system, how the separation process occurs, and the types of detectors commonly used. Below is a summary of important points to consider:
| Key Aspect | Description |
|---|---|
| Mobile Phase | The mobile phase is a carrier gas, often helium or nitrogen, which transports the sample through the column. |
| Stationary Phase | The column is coated with a stationary phase, typically a liquid or solid adsorbent, that interacts differently with the sample components. |
| Column | The column is a tube, usually made of stainless steel or glass, where the separation takes place. It can be packed or capillary type. |
| Detector Types | Common detectors include Flame Ionization Detectors (FID), Thermal Conductivity Detectors (TCD), and Mass Spectrometers (MS), each with specific sensitivities. |
| Applications | This technique is used for environmental testing, food and beverage quality control, drug testing, and petrochemical analysis. |
Understanding these elements is critical to performing accurate and efficient analyses. The choice of mobile phase, stationary phase, and detector will depend on the nature of the substances being analyzed, ensuring that the system provides the most relevant data for the intended purpose.
Role of Solvents in Chromatographic Processes
Solvents play a crucial role in separation techniques, acting as the mobile phase that carries the sample through the column. Their primary function is to facilitate the movement of the sample components while also influencing the efficiency and selectivity of the separation. By interacting with the sample and the stationary phase, solvents help to achieve the desired separation of complex mixtures.
The choice of solvent can significantly impact the performance of the technique. Factors such as polarity, viscosity, and volatility must be considered to ensure that the solvent supports the separation process effectively. Below is a table outlining some key characteristics of solvents used in separation methods:
| Solvent Property | Impact on Separation |
|---|---|
| Polarity | Polar solvents are used for polar samples, while non-polar solvents are used for non-polar compounds, affecting how substances interact with the stationary phase. |
| Viscosity | High viscosity solvents can slow down the movement of the sample, leading to longer analysis times and potential column overloading. |
| Volatility | Volatile solvents evaporate easily and are ideal for techniques where rapid separation and detection are required, such as in gas-based methods. |
| Solubility | The solvent must dissolve the sample components efficiently to ensure they can be carried through the column without losing their integrity. |
By understanding the impact of solvent properties on the separation process, analysts can select the most appropriate solvent for each application, optimizing both the speed and accuracy of the results.
Chromatography Column Design and Use
The design and selection of columns are fundamental in achieving efficient separation of compounds in analytical techniques. Columns act as the medium through which the sample passes, allowing interactions between the sample components and the stationary phase. These interactions are crucial for achieving the desired separation, and the design of the column can significantly influence the efficiency and resolution of the process.
Column length, diameter, and the type of material used for packing all play important roles in the separation process. The choice of these factors depends on the nature of the sample, the desired separation, and the sensitivity required. Below is a table summarizing key aspects of column design:
| Column Property | Impact on Separation |
|---|---|
| Length | Longer columns offer better separation efficiency but require longer run times. Shorter columns are faster but may sacrifice resolution. |
| Diameter | Larger diameter columns allow for higher sample capacity, while smaller diameter columns improve resolution and sensitivity. |
| Column Packing | The material used to pack the column affects the interactions between the sample and the stationary phase, influencing separation quality and speed. |
| Flow Rate | The rate at which the mobile phase moves through the column affects both the resolution and time required for separation. |
Understanding these key design elements allows for optimization of the column to suit specific applications, ensuring accurate and reproducible results in separation analyses. Whether for routine quality control or advanced research, column design remains a critical factor in achieving high-performance separations.
How to Interpret Chromatographic Results
Interpreting the results of a separation process is a critical step in understanding the composition of a sample. The data usually come in the form of a chromatogram, which displays the separation of various components over time. The key to effective interpretation lies in analyzing the peak patterns, retention times, and intensities, which provide insight into the chemical properties and concentrations of the substances present in the sample.
To begin with, each peak in a chromatogram represents a different compound, with the area under the peak correlating to the amount of that substance in the sample. Retention time, the time it takes for a compound to travel through the system, is used to identify the specific components. However, interpreting the results requires careful attention to several factors, including baseline noise, peak resolution, and possible overlap between different substances.
Here are some key points to consider when analyzing chromatographic data:
- Peak Identification: Compare the retention times of observed peaks with known standards to identify compounds.
- Peak Area: The area under each peak gives an indication of the concentration of the compound in the sample.
- Resolution: High-resolution peaks indicate well-separated compounds, while overlapping peaks suggest poor separation.
- Baseline Stability: A stable baseline ensures accurate peak integration and reliable data interpretation.
Accurate interpretation of chromatographic results requires a comprehensive understanding of the system’s behavior and the compounds involved. By considering these factors, you can extract meaningful information about the sample and assess the effectiveness of the separation process.
Types of Chromatographic Phases and Their Importance
In any separation process, the interaction between different phases plays a crucial role in how effectively components are separated. These phases are typically categorized into two main types: the stationary phase and the mobile phase. The choice of these phases influences the separation efficiency, speed, and resolution of the analysis. Understanding the properties of these phases is essential for selecting the optimal method for a specific application.
Stationary Phase
The stationary phase remains fixed in place within the separation system and interacts with the sample as it moves through the system. It can be solid or liquid, and its primary role is to slow down or retain specific components of the sample, allowing for separation based on differing affinities.
- Solid Stationary Phases: These are commonly used in adsorption techniques, where components are separated based on their ability to adhere to the stationary material.
- Liquid Stationary Phases: In some cases, the stationary phase is a thin liquid layer coated onto a solid support. This is typically seen in partition techniques where separation occurs based on solubility differences.
Mobile Phase
The mobile phase is the fluid that moves through the stationary phase, carrying the sample along with it. The mobile phase can be a gas or a liquid, depending on the separation technique being used. Its primary role is to carry the sample components through the system, allowing them to interact with the stationary phase at different rates, leading to their separation.
- Gas Mobile Phases: In gas-based separations, the mobile phase is typically an inert gas like helium or nitrogen that transports the sample through the stationary phase.
- Liquid Mobile Phases: In liquid separations, solvents or mixtures of solvents are used as the mobile phase to carry the sample through the column or other medium.
The interaction between the stationary and mobile phases is vital for achieving separation. The properties of both phases, such as polarity, viscosity, and chemical compatibility, must be carefully chosen to ensure efficient separation of the components. Understanding the importance of these phases allows for more effective and precise separation processes across a wide range of applications.
Chromatography Problems and Troubleshooting Tips
During any separation process, various challenges can arise, affecting the quality and efficiency of the results. Understanding common issues and knowing how to address them is crucial for ensuring optimal performance. This section will explore typical problems encountered and offer practical solutions for overcoming them.
Common Issues in Separation Processes
Several factors can cause issues in separation techniques, leading to poor resolution, incomplete separation, or inconsistent results. Identifying the root cause is essential for finding the correct solution. Below are some of the most common problems:
- Low Resolution: Insufficient separation of components can result from improper settings or incompatible phases.
- Peak Tailoring: When peaks in chromatograms are not sharp and exhibit tailing, it often indicates issues with the stationary phase or column overload.
- Irregular Retention Times: Variability in retention times can occur due to fluctuations in the mobile phase composition or system instability.
- Baseline Drift: A drifting baseline is a common problem caused by fluctuations in temperature, pressure, or solvent instability.
Troubleshooting Tips
Once the issue is identified, there are several steps that can be taken to resolve it. The following troubleshooting tips can help address the most common separation problems:
- Ensure Proper Calibration: Regularly calibrate the equipment to maintain accurate measurements and consistent performance.
- Optimize Mobile Phase Composition: Ensure that the mobile phase is properly prepared, and consider adjusting its polarity or flow rate for better separation.
- Check for Blockages: Inspect the column and system for any blockages or contamination that could be affecting flow or separation efficiency.
- Adjust Temperature and Pressure: In some cases, adjusting the temperature or pressure can help stabilize the process and improve separation.
- Reduce Sample Load: Overloading the system with too much sample can lead to poor separation. Reducing the sample volume or concentration may improve results.
By addressing these common issues with the appropriate troubleshooting techniques, it is possible to optimize the performance of the separation process and achieve more reliable and reproducible results.
Preparing for Multiple Choice Chromatography Questions
Multiple-choice questions are a common format for assessing knowledge of separation techniques. Preparing for this type of assessment requires a clear understanding of key concepts and the ability to identify the correct answer from a set of choices. In this section, we will explore strategies for approaching such questions effectively and tips for mastering the material.
Key Areas to Focus On
To succeed in answering multiple-choice questions, it is essential to focus on the most important aspects of the subject. Understanding the fundamentals, terminology, and specific methods used in separation techniques is crucial. Below are some areas to prioritize during your preparation:
- Basic Principles: Understand the core concepts of how components are separated, including the role of phases, solvents, and equipment.
- Different Separation Techniques: Be familiar with various types of separation methods, their applications, and their specific advantages and limitations.
- Instrument Settings: Know how changes in parameters like flow rate, temperature, and pressure affect separation efficiency and outcomes.
- Interpreting Data: Be able to analyze chromatograms, identify peak patterns, and understand how to draw conclusions from data provided.
Effective Strategies for Answering Questions
While studying, it is also beneficial to practice answering questions to improve your test-taking skills. Here are some strategies to help you succeed when faced with multiple-choice options:
- Read the Question Carefully: Make sure you understand what is being asked before reviewing the options. Pay attention to key terms and any qualifiers like “always,” “never,” or “most likely.”
- Eliminate Obvious Incorrect Answers: If you are unsure about the correct answer, start by eliminating the most clearly incorrect options. This increases your chances of selecting the right choice.
- Use Knowledge of Similar Topics: Some answers may be similar to concepts you have already studied. Use your understanding of related areas to help narrow down your choices.
- Double-Check Your Answer: Once you have selected an option, take a moment to review the question and your answer to ensure they align logically.
By focusing on these strategies and dedicating time to studying key concepts, you can approach multiple-choice questions with confidence and improve your overall performance.
Study Tips for Chromatography Exams
Preparing for an assessment on separation techniques requires more than just memorizing facts. A deep understanding of the principles, methods, and applications is essential to succeed. In this section, we will explore effective study strategies that will help you grasp key concepts, retain important details, and improve your overall performance in such evaluations.
Organize Your Study Sessions
Effective preparation starts with organizing your time and materials. Break down the topics into manageable sections and focus on one area at a time. By creating a study plan, you can ensure that you cover all important topics systematically without feeling overwhelmed.
- Create a Schedule: Plan your study time around the topics you find most challenging, leaving time for review and self-testing.
- Prioritize Key Concepts: Focus on understanding the fundamental principles of separation processes, the different types of phases, and the common equipment used.
- Use Study Aids: Utilize diagrams, flowcharts, and mnemonic devices to help reinforce your understanding of complex concepts.
Active Learning Techniques
Active engagement with the material will help you retain information more effectively. Instead of passively reading your notes, apply active learning strategies that challenge you to recall and apply the knowledge.
- Practice Problem Solving: Work through practice problems to apply theoretical knowledge to real-life scenarios. This helps you understand how to use the concepts in practical situations.
- Teach What You Know: Explaining concepts to a peer or even to yourself is a great way to reinforce what you’ve learned and identify any gaps in your understanding.
- Review Past Materials: Go through old notes and practice materials to see how different topics relate to each other and reinforce long-term retention.
Stay Calm and Confident
As the assessment approaches, it’s important to manage stress and stay confident in your preparation. Regular breaks, a balanced routine, and positive self-talk will help you approach your studies with a clear mind.
- Take Breaks: Break your study sessions into intervals and make time for relaxation to avoid burnout.
- Stay Hydrated and Rested: Ensure that you get enough sleep and maintain proper hydration to keep your brain sharp during study sessions and the assessment itself.
By following these study tips and committing to a structured approach, you can master the content and approach the test with confidence, knowing that you are well-prepared.
Resources for Further Learning
To enhance your understanding of separation techniques and deepen your knowledge, it’s important to explore a variety of educational resources. Whether you are looking for textbooks, online platforms, or practical guides, there are plenty of materials that can help solidify your grasp on key concepts and provide you with real-world applications. In this section, we will highlight some of the best resources for continuous learning in this field.
Books and Textbooks
Textbooks are an excellent source for building foundational knowledge. They often provide in-depth explanations, detailed examples, and comprehensive coverage of theoretical principles.
- “Introduction to Separation Processes” by James F. F. Smith: A comprehensive book that explains fundamental techniques and their applications in various industries.
- “Separation Process Principles” by J. D. Seader and Ernest J. Henley: This textbook offers a detailed exploration of the theory and practice behind separation methods.
- “Practical Guide to Laboratory Techniques” by David R. Lide: This resource focuses on practical techniques and troubleshooting tips for laboratory experiments.
Online Courses and Tutorials

Online platforms offer flexibility and access to expert instructors, allowing you to learn at your own pace. Interactive courses can guide you through both theoretical aspects and practical applications.
- Coursera – Separation Science Courses: Offers a range of courses from beginner to advanced levels, with a focus on both theory and practical techniques.
- Udemy – Separation Techniques in Chemistry: A collection of affordable courses that provide step-by-step guides on various separation methods and their uses in different industries.
- edX – Separation Technology: Free and paid courses from leading universities that dive deep into cutting-edge separation technologies and their real-world applications.
Scientific Journals and Articles
For more advanced learners or professionals in the field, scientific journals provide the latest research, innovations, and methodologies. Reading peer-reviewed articles can expose you to current trends and emerging techniques.
- Journal of Separation Science: A prominent journal dedicated to research in separation science, with articles on both theoretical developments and practical applications.
- Analytical Chemistry: Publishes research on analytical techniques, including advancements in separation methods and their integration into various industries.
- Trends in Analytical Chemistry: Focuses on the latest breakthroughs and trends in analytical technologies, with detailed research on innovative separation approaches.
Practical Guides and Web Resources
If you prefer hands-on learning, practical guides and websites with step-by-step instructions are invaluable. These resources can provide immediate insights into best practices and troubleshooting techniques.
- Labster – Virtual Labs: Provides virtual laboratory simulations where you can practice separation techniques in a controlled, virtual environment.
- Separation Science – Practical Tips: Offers articles, blogs, and case studies that discuss real-world applications and common challenges in separation processes.
- PubMed – Research Articles: A database of scientific articles that includes research on the latest methods, tools, and advancements in separation science.
By utilizing these diverse resources, you can continuously expand your expertise and stay up-to-date with the latest developments in this rapidly evolving field.