Organic Chemistry Exam Questions and Answers

Success in higher-level scientific assessments requires a deep understanding of core principles and the ability to apply knowledge effectively under pressure. Thorough preparation involves mastering various techniques, concepts, and problem-solving skills. Whether tackling theoretical problems or interpreting data, each section demands attention to detail and critical thinking.
With numerous topics to cover, it’s important to focus on the most commonly tested areas. Students should become familiar with the types of tasks they are likely to encounter and practice regularly. Key to excelling is learning to approach each problem methodically, breaking it down into manageable parts.
By developing strong analytical skills and ensuring a solid grasp of the fundamental principles, individuals can significantly improve their performance. With the right strategies and targeted practice, overcoming the challenges of these assessments becomes much more attainable.
Organic Chemistry Exam Questions and Answers
Preparing for a scientific evaluation requires a strategic approach to mastering a broad range of material. Focused practice and familiarity with typical topics can help students perform at their best. Knowing what to expect allows individuals to tackle each problem with confidence and precision, turning complex challenges into manageable tasks.
Identifying Key Concepts
Understanding fundamental principles is the first step toward success. Focus on the most relevant subjects that frequently appear in tests. Having a clear grasp of core ideas ensures that even unexpected scenarios can be addressed effectively. Practice problems are essential tools in solidifying knowledge and developing a strong foundation.
Approaching Data Interpretation
Mastering how to interpret data is equally important. Many assessments require the ability to analyze various scientific figures and results. Whether dealing with spectral data or reaction sequences, the ability to draw meaningful conclusions from raw information is crucial. By practicing interpretation skills, you can improve your ability to swiftly and accurately answer questions in high-pressure situations.
Essential Concepts for Organic Chemistry Exams
To succeed in a scientific evaluation, it is crucial to master the foundational principles that form the core of the subject. These fundamental ideas are the building blocks for solving complex problems. Understanding these concepts allows students to approach each task with a clear mindset, making it easier to navigate through different scenarios and interpretations.
Among the key topics, reaction mechanisms and molecular structure play a central role. A strong grasp of how molecules interact and transform is essential for tackling various challenges. Functional groups and their behavior are another vital area that must be studied thoroughly. These components frequently appear in problems, making them an integral part of effective preparation.
Additionally, mastering techniques such as stereochemistry and understanding how to analyze molecular symmetry can be highly beneficial. The ability to recognize patterns and predict outcomes based on these principles can significantly enhance problem-solving efficiency during assessments.
Commonly Asked Organic Chemistry Questions
When preparing for a scientific assessment, it’s important to focus on the areas most frequently tested. Many evaluations feature similar types of challenges, so identifying patterns in the types of problems that commonly arise can significantly improve your preparation. Understanding these common topics will allow you to approach your study sessions with purpose and efficiency.
The following table highlights some of the most frequently encountered topics, offering insight into the areas that are essential for success. By reviewing these key areas, you can ensure you’re well-prepared to tackle the most common problems that appear in assessments.
| Topic | Description |
|---|---|
| Reaction Mechanisms | Understanding how molecules react and transform through various steps. |
| Functional Groups | Familiarity with different molecular groups and their behavior in reactions. |
| Stereo Chemistry | Study of spatial arrangement of atoms and their effects on reactions. |
| Molecular Structures | Ability to analyze the structure and bonding of complex compounds. |
| Spectral Data | Interpreting data from techniques such as NMR and IR spectroscopy. |
Understanding Reaction Mechanisms for Exams
One of the most critical skills for succeeding in scientific assessments is understanding the step-by-step processes that govern molecular interactions. Reaction mechanisms describe how bonds are broken and formed during chemical transformations. A clear understanding of these mechanisms not only helps in solving problems but also provides insight into predicting the outcome of different reactions.
Key Steps in a Reaction Mechanism
Each transformation follows a series of steps, often involving the movement of electrons. Mastering these sequences requires familiarity with terms such as nucleophiles, electrophiles, intermediates, and transition states. Key steps include:
- Initiation: The starting point of the reaction where the first bond is broken.
- Propagation: The series of steps that continue the transformation, creating new bonds.
- Termination: The final stage where the reaction ends and stable products are formed.
Common Types of Reaction Mechanisms
There are several different types of mechanisms that frequently appear in assessments. Understanding the general principles of each can help in recognizing patterns in reaction behavior. Some of the most common types include:
- Substitution Reactions – A process where one atom or group is replaced by another.
- Elimination Reactions – Involves the removal of atoms or groups from a molecule, resulting in the formation of a double bond.
- Addition Reactions – Atoms or groups are added to a molecule, often across a double bond.
- Radical Reactions – Involves unpaired electrons, often initiated by light or heat.
By practicing these mechanisms and understanding their typical steps, you can approach each problem with a methodical mindset, increasing your chances of success during your assessment.
How to Approach Functional Groups Questions
When faced with challenges involving specific molecular components, it is essential to understand their unique properties and behaviors. These parts of molecules play a crucial role in determining reactivity and interactions with other substances. Recognizing these functional groups and knowing how to handle them is a key strategy for solving complex problems in assessments.
Identifying Functional Groups
The first step in tackling any problem involving molecular components is the ability to identify them quickly. These groups often have distinct structural features that make them easy to spot. Some of the most common include:
- Alcohols – Containing a hydroxyl group (-OH) attached to a carbon.
- Aldehydes – Featuring a carbonyl group (C=O) with a hydrogen atom attached.
- Carboxylic Acids – A carbonyl group bonded to a hydroxyl group (-COOH).
- Esters – Derived from an acid and an alcohol, with a structure of -COO-.
- Amines – Containing a nitrogen atom bonded to hydrogens or carbon groups.
Understanding the Behavior of Functional Groups
Once you have identified the relevant functional group, understanding its chemical properties is essential for solving related problems. These properties determine how the group interacts with other molecules and how it can be manipulated in reactions. For example, alcohols are often involved in substitution reactions, while carboxylic acids typically participate in esterification.
By recognizing the behavior of these components in different contexts, you can approach problems with greater confidence, predicting outcomes and applying the correct methods to find solutions.
Key Organic Reactions to Remember
Understanding the fundamental processes that govern molecular transformations is essential for excelling in scientific assessments. Certain reactions appear frequently, making it crucial to recognize and master them. By focusing on these key processes, students can approach their preparation more effectively and gain a deeper understanding of how different substances interact.
Common Reaction Types
Several reaction types form the basis for solving many problems. These transformations are characterized by the breaking and forming of chemical bonds, often resulting in the creation of new compounds. Some of the most important reactions to remember include:
- Substitution Reactions – One atom or group is replaced by another in a molecule.
- Elimination Reactions – Atoms or groups are removed, leading to the formation of a double bond.
- Addition Reactions – New atoms or groups are added to a molecule, often involving unsaturated compounds.
- Condensation Reactions – Two molecules combine, releasing a small molecule like water or alcohol.
Reaction Mechanisms to Focus On
Each reaction type follows a specific sequence of events, known as a reaction mechanism. Understanding these steps can help you predict the outcome of a reaction and apply the correct strategy to solve problems. Some key mechanisms include:
- Radical Reactions – Involve the transfer of electrons to form highly reactive intermediates.
- Nucleophilic Substitution – A nucleophile replaces a leaving group in a molecule.
- Electrophilic Addition – An electrophile adds to an electron-rich site in a molecule.
By mastering these key reactions and their mechanisms, you will be better prepared to identify patterns and solve problems effectively during your assessment.
Practice Problems for Organic Chemistry Exams
To reinforce understanding and improve problem-solving abilities, regular practice is essential. Working through a variety of problems allows you to apply theoretical knowledge in practical situations, enhancing both speed and accuracy. It also helps identify areas of weakness that require further attention, ensuring more effective preparation for assessments.
Types of Practice Problems
There are several types of challenges that often appear in scientific evaluations. These problems may focus on different aspects of molecular interactions, from basic structural analysis to complex reaction mechanisms. Some common types include:
- Mechanism Problems – Determine the steps involved in a chemical transformation.
- Synthesis Problems – Design a pathway to synthesize a particular compound from available reactants.
- Identification Problems – Identify functional groups, molecular structures, or reaction products based on given data.
- Data Interpretation Problems – Analyze spectroscopic data, such as NMR, IR, or mass spectrometry, to deduce molecular structures.
Benefits of Regular Practice
By consistently solving problems of varying difficulty, you can build a strong foundation in the subject. Practice enhances familiarity with common patterns, speeds up your problem-solving process, and boosts confidence during assessments. It also helps develop a deeper understanding of how different concepts are interconnected, enabling more effective application of knowledge.
Tips for Memorizing Organic Chemistry Structures
Memorizing molecular structures can be a daunting task, but with the right strategies, it becomes much more manageable. Understanding the relationships between atoms and how they form specific arrangements is key to visualizing these structures. The goal is to develop both familiarity and confidence, enabling you to quickly recognize and recall complex molecules during assessments.
Effective Memorization Techniques
There are several methods that can help make memorizing molecular structures easier. By incorporating these strategies into your study routine, you can enhance retention and improve recall during tests:
- Chunking – Break down larger structures into smaller, manageable parts, focusing on common substructures that appear frequently.
- Use Visual Aids – Drawing structures repeatedly or using molecular models helps reinforce spatial understanding.
- Create Flashcards – Use flashcards to quiz yourself on structures, functional groups, and bonding patterns. Visual cues are powerful memory aids.
- Relate Structures to Functions – Understand the function of each group or molecule, as this often helps link structure with purpose.
- Practice Regularly – The more you practice drawing and identifying structures, the more familiar they will become, improving retention over time.
Making the Most of Study Sessions
Incorporating these techniques into your study schedule is essential for long-term retention. Consistent, spaced-out practice will ensure that these structures become ingrained in your memory. Additionally, grouping related structures together, such as various functional groups or reaction intermediates, can help make memorization more efficient and reduce confusion during assessments.
Mastering Synthesis Reactions for the Exam
One of the most important skills in a scientific assessment is the ability to design pathways for creating specific compounds from simpler starting materials. Synthesis reactions are a critical part of the process, involving a series of chemical transformations that lead to the desired product. Mastering these reactions requires a clear understanding of reagents, reaction conditions, and the types of transformations that can occur.
Key Strategies for Success
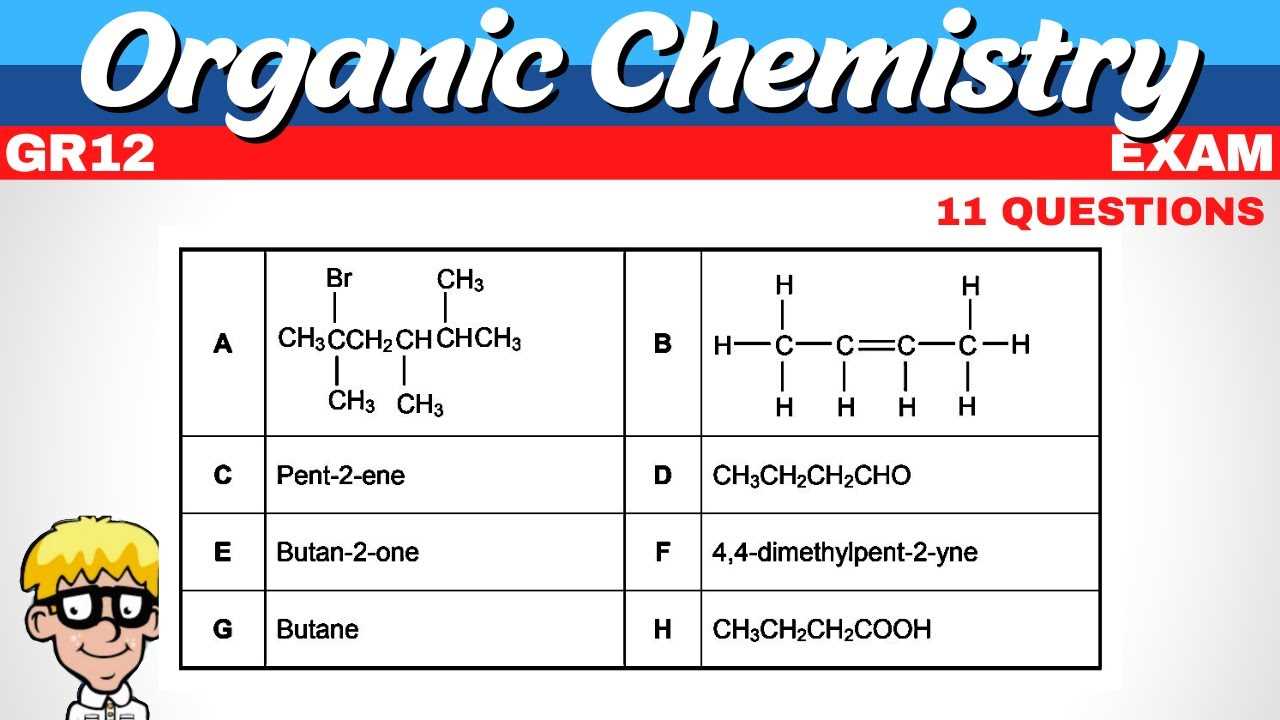
To excel in synthesis problems, it’s essential to approach them systematically. Recognizing the various reaction types and their specific conditions will allow you to select the best reagents and methods for each scenario. Here are some key strategies for mastering synthesis reactions:
- Understand Functional Group Reactivity – Each functional group behaves differently under specific conditions, so knowing how they react in various environments is crucial.
- Plan Step-by-Step – Break down the reaction into smaller, manageable steps. This helps identify the correct sequence of reactions needed to achieve the desired product.
- Know Common Reagents – Familiarize yourself with reagents that are commonly used in synthesis, as well as the conditions under which they work best.
- Use Retrosynthesis – Work backward from the target molecule to determine the simplest starting materials and the most efficient pathway.
Example Synthesis Pathways
Reviewing some common synthesis pathways can provide useful insight into how reactions can be combined to create complex molecules. The following table outlines a few examples of common transformations and their corresponding reactions:
| Target Molecule | Starting Material | Reaction Type |
|---|---|---|
| Alcohol | Alkene | Hydration |
| Aldehyde | Alcohol | Oxidation |
| Amine | Halide | Nucleophilic Substitution |
| Ketone | Alkene | Hydroformylation |
By familiarizing yourself with these pathways and practicing various synthesis problems, you can confidently approach any challenge involving compound formation in your assessments.
Common Mistakes in Organic Chemistry Exams
Even the most prepared students can make errors during assessments. Recognizing the types of mistakes that frequently occur helps to avoid them and ensures better performance. These common pitfalls can arise from misunderstandings of fundamental concepts, misinterpreting the problem, or overlooking key details in the process. Being aware of these mistakes allows students to approach problems more carefully and systematically.
Frequent Mistakes to Avoid
There are several recurring errors that students often make when dealing with problems related to molecular reactions and structures. By understanding these mistakes, you can improve your problem-solving strategies and reduce the likelihood of missteps. Some of the most common mistakes include:
- Ignoring Reaction Conditions – Not paying attention to temperature, solvent, or catalysts can lead to incorrect predictions about how a reaction will proceed.
- Misidentifying Functional Groups – Failing to recognize or correctly identify functional groups can cause incorrect assumptions about how a molecule will behave in a reaction.
- Incorrect Mechanism Prediction – Assuming the wrong reaction mechanism or sequence of steps is a frequent mistake, especially in complex transformations.
- Overlooking Stereochemistry – Neglecting to account for chirality or stereochemical configurations can lead to errors, especially in reactions involving asymmetric molecules.
- Rushing Through Drawings – Incomplete or incorrect structure drawings often occur when students rush through molecular diagrams without checking their work carefully.
How to Prevent These Mistakes
Taking a methodical approach to each problem can help prevent many of these errors. Here are some tips to ensure a more accurate and efficient approach:
- Double-check functional groups and ensure their correct identification before proceeding with any reaction steps.
- Carefully read the instructions and pay close attention to the conditions specified for each reaction.
- Take your time with structure drawings and mechanisms to avoid making careless mistakes.
- Review the key principles of stereochemistry to ensure proper consideration of all spatial arrangements.
- Practice a variety of problems to become familiar with common scenarios and reaction pathways.
By avoiding these common errors and focusing on the details, you can improve your performance and confidence in problem-solving during assessments.
How to Tackle Stereochemistry Questions
Understanding molecular spatial arrangements is crucial when solving problems related to the three-dimensional structure of molecules. These questions often involve concepts such as chirality, isomerism, and the impact of stereochemical configurations on reactivity and physical properties. Mastering these topics requires a clear grasp of how atoms and groups are positioned in space and how their interactions influence molecular behavior.
Key Concepts to Remember
Before diving into problems, it’s essential to understand the fundamental concepts involved in stereochemistry. Here are some key areas to focus on:
- Chirality – A molecule is considered chiral if it cannot be superimposed on its mirror image. Identifying chiral centers (often carbon atoms bonded to four different groups) is crucial in many stereochemical problems.
- Enantiomers – These are pairs of molecules that are mirror images of each other but cannot be superimposed. Understanding how to identify and differentiate between enantiomers is vital.
- Diastereomers – These are stereoisomers that are not mirror images of each other. Unlike enantiomers, diastereomers have different physical properties, which can be used to distinguish them.
- R/S Configuration – The Cahn-Ingold-Prelog system is used to assign the absolute configuration of chiral centers. Being familiar with this system is essential for determining the stereochemical arrangement of molecules.
Strategies for Solving Stereochemistry Problems
Once you have a solid understanding of the key concepts, follow these strategies when approaching stereochemical problems:
- Identify Chiral Centers – Look for carbon atoms attached to four different substituents, as these are typically the centers of chirality.
- Assign Priority – Use the Cahn-Ingold-Prelog rules to assign priority to substituents based on atomic number and the sequence of atoms attached to the chiral center.
- Use Models – If you’re struggling to visualize 3D structures, consider using molecular models or drawing the molecules in three dimensions to better understand the spatial arrangements.
- Practice Rotation and Mirror Images – Practice determining whether two molecules are mirror images or if they can be superimposed, as this is often a key part of stereochemistry questions.
- Pay Attention to Symmetry – In some cases, molecules may appear to have chiral centers, but symmetry may make them achiral overall. Carefully check for plane of symmetry or other structural features.
By mastering these techniques, you can approach stereochemical questions with confidence, ensuring a thorough understanding of molecular structures and their implications in reactions.
Understanding NMR Spectroscopy in Exams
Interpreting spectroscopic data is a crucial skill when solving problems related to molecular structure. One of the most commonly used techniques for determining molecular composition and connectivity is Nuclear Magnetic Resonance (NMR) spectroscopy. This tool provides valuable information about the hydrogen and carbon environments within a compound, allowing for the deduction of its structure. Mastering the interpretation of NMR spectra is essential for tackling related problems effectively during assessments.
Key Principles of NMR Spectroscopy
To interpret NMR spectra correctly, it is important to understand how different signals correspond to various structural features. Here are the main components to focus on when studying NMR data:
- Chemical Shifts – The position of the peaks on the NMR spectrum (measured in ppm) reveals information about the electronic environment of hydrogen or carbon atoms. Electronegativity, nearby functional groups, and hybridization influence these shifts.
- Spin-Spin Coupling – The splitting of signals, also known as multiplets, occurs due to interactions between non-equivalent hydrogen atoms. The number of splits and their pattern can provide details about the number of neighboring hydrogen atoms.
- Integration – The area under each peak corresponds to the relative number of nuclei (hydrogens or carbons) contributing to that signal. This information helps determine the ratio of atoms in different environments.
- Coupling Constants – The distance between split peaks in a multiplet (measured in Hz) can reveal information about the coupling between neighboring atoms and their spatial relationship.
How to Approach NMR Spectra Interpretation
When presented with NMR spectra in assessments, follow these steps to interpret the data systematically:
- Identify the Number of Peaks – Each distinct environment (hydrogen or carbon) will generate a unique signal. Count the peaks to determine the number of different environments in the compound.
- Analyze Chemical Shifts – Compare the observed chemical shifts with known ranges for various functional groups and atom types. This will help assign environments to specific atoms.
- Determine Multiplets – Observe the splitting of peaks to understand the number of adjacent hydrogens and the coupling patterns. Use this to deduce the connectivity between atoms.
- Integrate the Spectrum – Use the integration values to deduce the relative number of atoms in each environment, helping to establish the molecular formula.
With practice, interpreting NMR spectra becomes easier, allowing you to confidently identify structural features and solve related problems with accuracy.
How to Interpret Mass Spectrometry Data

Mass spectrometry is a powerful analytical technique used to determine the molecular mass and structure of compounds. By measuring the mass-to-charge ratio of ions, it provides insight into the molecular composition, fragmentation patterns, and isotopic distribution of a sample. Being able to interpret mass spectrometry data effectively is essential for identifying compounds and understanding their structure in detail.
Key Elements of Mass Spectrometry Data
When analyzing mass spectrometry data, several key features should be considered to extract meaningful information. Here are the main components to focus on:
- Molecular Ion Peak – The molecular ion (M+) represents the intact molecule, giving its molecular weight. This peak is typically the highest m/z value in the spectrum and corresponds to the original compound.
- Fragmentation Peaks – The spectrum often shows peaks corresponding to fragments of the parent molecule. These fragments result from the molecule breaking apart under the influence of high-energy electrons, and their pattern can help deduce the structure.
- Isotope Peaks – Some elements, like chlorine or bromine, have isotopes with different masses. These create additional peaks that appear alongside the molecular ion, which can aid in identifying certain elements in the compound.
- Base Peak – The base peak is the most intense peak in the spectrum and represents the most abundant ion. It is often used as a reference point when analyzing the data.
How to Analyze Mass Spectrometry Data
To interpret mass spectrometry data systematically, follow these steps:
- Locate the Molecular Ion – Identify the peak corresponding to the intact molecule to determine the molecular weight of the compound.
- Examine Fragmentation Patterns – Look for the most common fragments and their corresponding m/z values. These can give clues about the structure and functional groups present in the compound.
- Check for Isotopic Patterns – Determine the presence of isotopic peaks, especially for elements like chlorine (Cl) or bromine (Br), to confirm the elemental composition of the compound.
- Analyze the Base Peak – Understand the base peak, as it reveals the most abundant fragment, which can be useful for determining the most stable part of the molecule.
With practice, interpreting mass spectrometry data becomes a powerful tool in the identification and structural analysis of molecules, offering invaluable insights into their composition.
Preparing for Multiple Choice Assessments
Multiple choice assessments can be a challenging yet effective way to test your understanding of key concepts. These tests often cover a broad range of topics, requiring both a deep grasp of material and the ability to quickly apply knowledge. Success in these types of questions comes from preparation, strategy, and careful reading of each option.
Effective Study Strategies
To perform well on multiple choice questions, it’s crucial to employ targeted study strategies. Here are some key techniques to help you prepare:
- Focus on Core Concepts – Make sure you have a strong understanding of the fundamental principles. Knowing the basics allows you to reason through more complex questions and eliminate incorrect choices.
- Use Practice Questions – Familiarize yourself with the format and types of questions by practicing with previous tests or sample problems. This will help you get comfortable with the style and time constraints.
- Make Study Guides – Condense large amounts of material into summaries or flashcards. This allows for quick revision and helps you retain key points for multiple choice testing.
- Review Mistakes – When practicing, make sure to thoroughly review your mistakes. Understanding why a particular answer was wrong will help you avoid similar errors during the actual test.
Strategic Test-Taking Tips
Once you’re ready for the assessment, it’s important to approach each question with strategy. Follow these tips during the test:
- Read Each Question Carefully – Make sure you fully understand what each question is asking before considering the answer choices. Misinterpreting the question can lead to unnecessary mistakes.
- Eliminate Clearly Incorrect Options – Often, you can immediately rule out one or more answers that are clearly wrong, narrowing your choices and increasing the likelihood of selecting the correct answer.
- Watch for Traps – Be cautious of answer choices that look plausible but are subtly incorrect. Multiple choice tests often include “distractors” designed to confuse.
- Trust Your First Instinct – If you are unsure about an answer, your first choice is often the best. Second-guessing yourself can lead to unnecessary errors.
By incorporating these strategies into your study routine and test-taking approach, you can maximize your performance on multiple choice assessments and tackle each question with confidence.
Time Management Strategies for Assessments
Effective time management is crucial when preparing for and taking tests. Properly allocating your time during both study sessions and the actual assessment ensures that you can cover all necessary material and complete each task without rushing. By planning ahead and using time wisely, you can improve your performance and reduce stress.
During preparation, focus on creating a structured schedule that allows enough time for review while balancing other commitments. Break your study sessions into manageable chunks, with designated breaks to avoid burnout. During the assessment itself, time management becomes even more critical as it determines how effectively you can complete the entire test.
Here are several strategies to help you manage your time effectively:
- Prioritize High-Impact Topics – Identify areas that are likely to carry the most weight in the test and prioritize those during study sessions. Focusing on key concepts and difficult topics will help you maximize your efforts.
- Practice with Timed Tests – Simulate test conditions by practicing with timed quizzes or mock assessments. This helps you get a sense of how long to spend on each question and builds confidence.
- Set Realistic Time Limits – During the test, allocate a specific amount of time to each section or question. If you’re stuck on one, move on and return to it later to ensure you don’t run out of time for easier questions.
- Don’t Overthink – If a question is taking too long to answer, it may be better to make an educated guess and move on. Overthinking can waste precious time and lower your chances of completing all sections.
By employing these time management techniques, you can increase your efficiency, reduce anxiety, and improve your overall performance on tests. Balancing your preparation time wisely and managing your pace during the test is key to achieving success.
Top Resources for Test Preparation
When preparing for a challenging assessment, utilizing the right resources can make all the difference in understanding key concepts and mastering the material. A combination of textbooks, online platforms, practice problems, and study groups can provide comprehensive support to enhance your preparation. Choosing the most effective tools for your study style will help you optimize your learning process and build confidence for the test.
Here are some of the top resources to consider for your test preparation:
- Textbooks – Trusted textbooks provide detailed explanations, practice exercises, and example problems. Look for those that explain concepts clearly and offer ample practice problems to test your knowledge.
- Online Study Platforms – Websites and apps like Quizlet, Khan Academy, or Coursera offer interactive lessons, practice quizzes, and video tutorials. These can help you grasp complex topics and reinforce learning.
- Practice Tests – Completing full-length practice tests under timed conditions can help you gauge your readiness and familiarize yourself with the structure and pacing of the assessment. Websites and study books often offer mock tests and sample questions.
- Flashcards – Using flashcards for memorization can help reinforce important definitions, mechanisms, or structures. Flashcards are particularly useful for quick, repetitive learning.
- Study Groups – Joining a study group provides the opportunity to discuss difficult concepts, quiz each other, and learn through collaborative discussion. Teaching others what you’ve learned can also reinforce your own understanding.
- Video Tutorials – Platforms like YouTube or educational websites offer video explanations of tough topics. Visual learning through animations or step-by-step breakdowns can make complex ideas easier to understand.
By leveraging these resources, you can ensure thorough preparation and a deeper understanding of the material. Combining multiple tools–such as textbooks, online platforms, and practice problems–will help you cover all aspects of the subject and approach the test with confidence.