Flame Test Lab Analysis Answers and Insights

When working with various substances, determining their composition is crucial for a wide range of scientific, industrial, and educational purposes. Through specific procedures, experts can observe and interpret the distinctive properties of materials, helping to classify them based on their chemical makeup. This process is essential for industries such as manufacturing, chemistry, and environmental science.
By utilizing specific techniques that involve applying heat and observing the reactions of materials, professionals can reveal important characteristics that are otherwise hidden. The results obtained from these experiments provide valuable insights into the presence of certain elements, offering a clear understanding of the material’s structure and behavior under different conditions.
In this article, we will delve into the methodologies employed in these procedures, the types of materials typically analyzed, and the key takeaways from the results. Understanding these concepts can greatly enhance our ability to work with diverse substances and make informed decisions in various applications.
Flame Test Lab Analysis Answers
In various scientific disciplines, identifying the composition of materials is a fundamental task. Specialized methods allow researchers to observe the behavior of substances when subjected to specific conditions, providing crucial insights into their chemical makeup. This process helps reveal the presence of particular elements, making it possible to distinguish between different compounds and understand their properties.
One of the key aspects of this method is the color emitted by materials when exposed to high temperatures. Each element reacts in its own unique way, producing a characteristic hue that serves as an indicator of its identity. By observing these reactions, scientists can quickly and accurately determine the elements present in an unknown sample.
The results obtained from these procedures are essential in many fields, from academic research to industrial applications. They help inform decisions in product development, quality control, and environmental monitoring, making this technique invaluable in various industries. Understanding how to interpret these reactions and the information they provide is key to harnessing the full potential of this method.
Understanding the Flame Test Method
This method is a simple yet effective approach used to identify the elements present in a substance based on their reaction to heat. When exposed to high temperatures, different materials produce distinct colors, which are the result of specific light emissions from the atoms as they become energized. By observing these reactions, it is possible to identify various components in a sample, without the need for complex or expensive equipment.
The principle behind this technique is rooted in atomic behavior. When a substance is heated, the electrons within its atoms absorb energy and move to higher energy levels. As they return to their original state, they release energy in the form of visible light. Each element emits light at characteristic wavelengths, creating a unique spectral pattern that can be linked to a specific element.
This method is commonly employed in many educational settings and is also utilized in industries where quick and straightforward identification of materials is needed. Its simplicity and efficiency make it an accessible and valuable tool for those working with chemical substances, providing rapid insights into their composition.
Key Principles of Flame Test Analysis
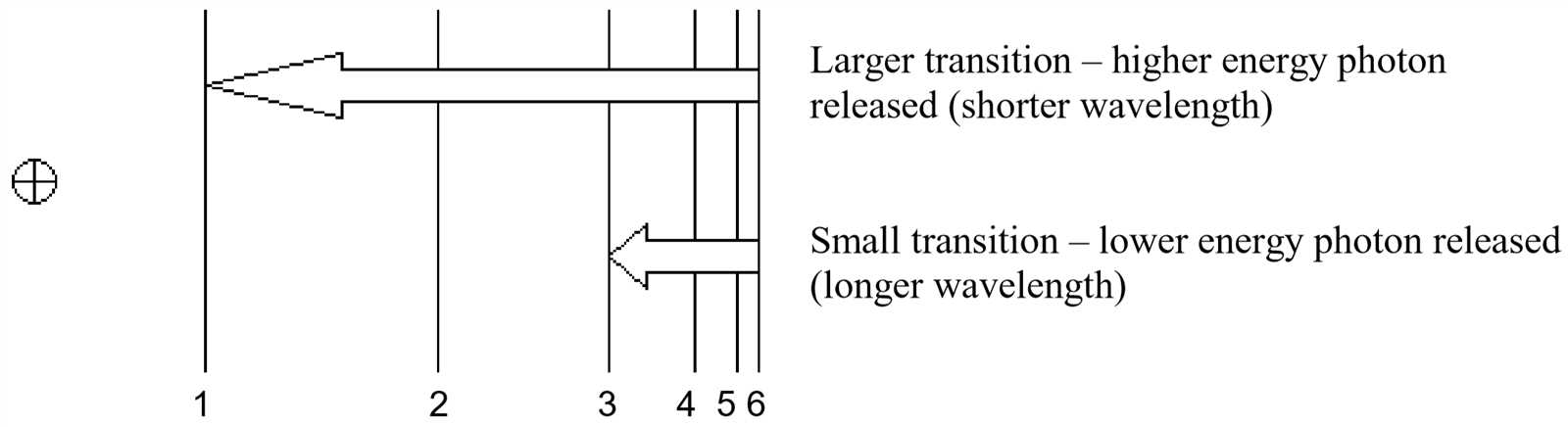
The process of identifying materials by observing their reaction to heat is based on a few fundamental principles of physics and chemistry. When a substance is heated, its atoms absorb energy, which causes electrons to move to higher energy levels. As the electrons return to their normal state, they release energy in the form of light, which can be observed and measured. The color of the emitted light depends on the specific energy levels of the element’s electrons, making this method useful for distinguishing different elements.
Each element emits light at characteristic wavelengths, which corresponds to a unique color. This distinctive color is the key to identifying the element present in the sample. By observing the color produced during the heating process, researchers can determine the composition of an unknown material. The color emitted is directly linked to the atomic structure of the element and its electron transitions.
| Element | Color Emitted |
|---|---|
| Sodium | Yellow |
| Potassium | Lilac |
| Calcium | Orange-red |
| Copper | Green |
This fundamental concept makes it possible to analyze materials quickly and effectively, providing valuable information in fields such as chemistry, forensics, and environmental science. Understanding the key principles of this method enables practitioners to gain insights into the material composition based on light emissions, which is a direct reflection of the substance’s atomic structure.
What Flame Test Reveals About Materials
This method provides valuable insights into the composition of materials by observing their behavior under heat. When subjected to high temperatures, substances release light at specific wavelengths, which corresponds to particular elements within the material. This reaction can help reveal the presence of certain metals and other components that are otherwise difficult to identify through conventional methods.
Through this process, it is possible to determine a wide range of properties and characteristics about a material, including:
- Elemental Composition: By analyzing the colors emitted, researchers can identify the specific elements present in a sample.
- Purity of the Sample: Variations in the intensity or shade of the emitted light can indicate the level of purity of the substance.
- Presence of Contaminants: The emission of unexpected colors can signal the presence of other substances or impurities mixed with the material.
- Material Type: The type of material, such as whether it is an oxide or a salt, can be inferred based on the reaction observed.
In essence, this method allows experts to gather detailed information about a material’s atomic structure and composition, helping to identify both its basic elements and any potential contaminants. It serves as a quick, cost-effective tool for both academic research and practical applications in various industries.
Common Applications of Flame Test Results
The results obtained from heating substances to observe their reactions can be applied across various fields, providing essential information about the materials being examined. These observations are particularly valuable in identifying elements and compounds in situations where quick and accurate analysis is necessary. The method offers a simple yet effective way to reveal the composition of substances, making it useful in both research and industrial settings.
Educational Purposes
This technique is widely used in educational settings to demonstrate the principles of atomic structure and electron behavior. It offers a hands-on approach for students to observe the unique color emissions of different elements, helping them understand complex chemical concepts in a visual and engaging way. Teachers use this method to explain the basics of atomic theory, making abstract ideas more accessible and relatable.
Industrial and Environmental Applications
In industrial applications, particularly in manufacturing and material testing, this method is utilized to identify elements in raw materials, ensuring that they meet quality standards. It also plays a crucial role in environmental science, where it can be used to detect trace amounts of metals and contaminants in soil, water, and air samples. Industries such as metallurgy, waste management, and water treatment rely on this technique for quick and efficient elemental analysis.
How Flame Test Detects Metal Elements
This method is particularly useful for detecting the presence of metal elements in a material by observing the specific colors emitted when the substance is exposed to heat. Each metal element has its unique set of electron energy levels, which means that when it is heated, it releases light at characteristic wavelengths. These wavelengths correspond to specific colors that can be identified and matched to particular metals.
Principle of Emission Spectra
When a metal is heated, its atoms absorb energy, causing electrons to jump to higher energy levels. As these electrons return to their ground state, they release energy in the form of visible light. The wavelength, or color, of the emitted light is specific to each element, which is why this method is so effective for identifying different metals. By carefully observing the color produced, one can determine the identity of the metal present in the sample.
Common Metal Elements Detected
Several metals are commonly identified using this technique due to their distinct emission colors. Some of these include:
- Sodium: Emits a bright yellow color
- Potassium: Produces a light violet or lilac hue
- Calcium: Releases an orange-red glow
- Copper: Shows a green or blue-green light
- Strontium: Gives off a crimson red color
By recognizing these characteristic colors, scientists and researchers can quickly and accurately detect metal elements in unknown substances, making this method a valuable tool in various applications such as material testing and environmental analysis.
Challenges in Flame Test Lab Procedures
While this method is widely used for identifying elements, there are several challenges that can arise during the process. Despite its simplicity, factors such as interference from impurities, inaccurate observations, and limitations in the sensitivity of the equipment can affect the reliability of the results. These challenges need to be addressed carefully to ensure accurate and consistent outcomes, especially when identifying trace amounts of elements or working with complex mixtures.
Interference from Impurities
One of the primary challenges is the presence of impurities in the sample. When other elements or compounds are mixed with the substance being analyzed, they can emit their own characteristic colors, which may overlap with the emission of the target element. This can make it difficult to isolate the specific color associated with the element of interest, leading to potential misidentifications.
Limited Sensitivity and Resolution
Another limitation is the sensitivity of the method. While it is effective for identifying larger concentrations of elements, it may not be reliable for detecting trace amounts. The color produced by small amounts of an element may not be intense enough to be clearly visible, or it may be obscured by the emission from more abundant substances in the sample.
| Common Challenges | Potential Solutions |
|---|---|
| Presence of Impurities | Purify samples and use more controlled environments to minimize contamination. |
| Overlapping Emissions | Utilize advanced filters or spectral analysis tools to isolate specific wavelengths. |
| Low Sensitivity | Increase the intensity of the heat source or use more sensitive detection equipment. |
Overcoming these challenges requires careful preparation and proper equipment. By addressing these issues, the reliability and effectiveness of this technique can be significantly improved, making it a more valuable tool for elemental identification in various fields.
Interpreting Color Changes in Flame Tests
When a substance is heated, it emits light at specific wavelengths, producing characteristic colors that correspond to the elements within the sample. The color changes observed during this process provide crucial information for identifying the material’s composition. By carefully observing these color shifts, one can determine the presence of particular elements and distinguish between different substances. However, interpreting these changes requires understanding the relationship between the elements’ energy levels and the emitted wavelengths.
The colors emitted during heating are the result of electrons within atoms absorbing and then releasing energy as they return to lower energy states. Each element has its unique set of energy levels, which is why each produces a distinct color when subjected to heat. The process of interpreting these color changes involves matching the observed hues to known emissions from specific elements.
Common Element Emission Colors
Each metal element produces a specific color when heated. Here are some common elements and the colors they emit:
- Sodium: Bright yellow
- Potassium: Light violet or lilac
- Calcium: Orange-red
- Copper: Green
- Strontium: Red
- Barium: Pale green
Factors Affecting Color Interpretation
While color identification can be straightforward, certain factors may influence the interpretation of the results:
- Intensity: The brightness of the color can vary depending on the concentration of the element in the sample. A faint color might indicate a low concentration, while a bright color suggests a higher concentration.
- Contaminants: The presence of other substances may introduce additional colors, which can obscure or alter the observed emission. Proper sample preparation is crucial to minimize this effect.
- Temperature: The temperature applied during the heating process can affect the intensity and clarity of the emitted light. Higher temperatures often produce more intense colors.
Understanding these factors helps ensure accurate interpretation of the color changes, providing valuable insights into the elements present in the sample. Proper observation and comparison with known reference colors are key to achieving reliable results.
Role of Flame Test in Chemical Identification
The technique of heating substances to produce visible color emissions plays an essential role in the identification of chemical elements within compounds. By observing the colors emitted when materials are exposed to high heat, one can quickly determine the presence of specific metals and other components. This method is particularly useful in fields such as chemistry, environmental science, and manufacturing, where rapid elemental identification is crucial for further analysis or processing.
The ability to observe distinct colors allows scientists to differentiate between various elements based on their characteristic emission spectra. This approach is often used in preliminary tests to narrow down the possible composition of a sample before more detailed analysis is conducted. It offers a fast, straightforward way to identify the major components of unknown substances, making it a valuable tool in chemical investigations.
Benefits of Using Flame Emission for Chemical Identification
Several advantages make this technique a preferred method for chemical identification:
- Speed: The procedure is quick, allowing for immediate results when time is of the essence.
- Cost-effective: It requires minimal equipment and is less expensive than more advanced spectroscopic techniques.
- Simplicity: The method is straightforward and can be performed with relatively little training.
- Non-destructive: The substance remains intact after the process, making it possible to use the sample for further analysis if needed.
Applications in Chemical Identification
This method is employed in various sectors for elemental analysis, including:
| Industry | Application |
|---|---|
| Chemical Manufacturing | Identification of raw materials and quality control |
| Environmental Science | Detection of metals and pollutants in soil and water |
| Forensic Science | Analysis of trace elements in criminal investigations |
| Education | Teaching basic chemical principles and atomic theory |
The ability to quickly identify chemical elements through color emissions is invaluable across numerous scientific fields. It enhances research efficiency, aids in quality control, and supports environmental monitoring efforts, among other uses.
Types of Materials Tested in Flame Tests
This method is commonly used to identify the elemental composition of various materials by observing the color emitted when they are subjected to intense heat. While it is primarily used for detecting metals, the technique can also be applied to certain non-metallic substances. The process helps distinguish different elements and compounds based on the specific light they emit when heated to high temperatures. It is widely used in educational, industrial, and research settings to determine the chemical makeup of a sample quickly and efficiently.
Materials typically tested in this method include metals, salts, and certain compounds that exhibit distinct and recognizable emission colors when exposed to heat. This process is particularly useful for identifying substances in qualitative analysis and when working with unknown or complex mixtures.
Metals and Metal Salts
One of the most common applications is identifying metals and their salts. When metals are heated, they produce specific flame colors that are unique to each metal. Some of the most frequently tested metals and their corresponding colors include:
- Sodium: Bright yellow
- Potassium: Lilac
- Calcium: Orange-red
- Copper: Green
- Barium: Pale green
- Strontium: Red
Non-Metallic Compounds
While metals are the primary focus, certain non-metallic compounds can also be tested. These compounds may release characteristic colors due to the presence of specific elements like halogens or phosphates. Some examples include:
- Phosphates: Yellow or green
- Chlorides: Green to blue
- Oxides: Various hues depending on the specific element involved
Testing non-metallic compounds can be especially useful in chemical research and environmental studies where the identification of various substances is critical. Understanding the emission spectra of these compounds allows scientists to analyze their composition and determine their properties.
Flame Test vs Other Analytical Methods
When it comes to identifying elements and compounds, there are a variety of techniques that can be employed. Each method has its own strengths and weaknesses, depending on factors such as the nature of the sample, the level of precision required, and the resources available. While certain methods can provide highly detailed information, others offer quick and cost-effective solutions for preliminary identification. This section explores how the technique of observing color emissions compares with other common analytical methods in terms of speed, accuracy, and practicality.
Among the most well-known methods for chemical identification are spectroscopy, chromatography, and gravimetric analysis. While each of these methods is widely used for specific purposes, the color emission technique offers distinct advantages in certain contexts.
Comparison Table of Analytical Methods
| Method | Strengths | Weaknesses | Typical Use |
|---|---|---|---|
| Color Emission | Quick, cost-effective, simple to perform | Less precise for complex samples | Preliminary identification of metals |
| Spectroscopy | Highly accurate, can detect trace elements | Expensive, requires specialized equipment | Detailed elemental analysis |
| Chromatography | Can separate complex mixtures, highly accurate | Time-consuming, requires preparation | Separation of chemical mixtures |
| Gravimetric Analysis | Highly precise, direct measurement | Slow, requires large sample sizes | Quantitative analysis of compounds |
The color emission method stands out for its speed and simplicity, making it particularly useful in educational settings or when quick results are needed. However, for more detailed and precise chemical analysis, techniques like spectroscopy or chromatography may be more appropriate. In cases where quantitative data is required, gravimetric analysis may be preferred despite its slower process.
In conclusion, the choice of analytical method depends on the specific requirements of the analysis, including factors like the complexity of the sample and the level of detail needed. Each method has its place in scientific and industrial applications, with the emission technique serving as an excellent first step in the identification process.
Flame Test Accuracy and Limitations
The method of observing color changes when a substance is heated is widely regarded for its simplicity and speed. However, like any scientific technique, it has its limitations, especially when it comes to accuracy and precision. While it provides quick qualitative results, it does not offer the same level of detail or quantitative data as other advanced methods. Understanding both its strengths and shortcomings is crucial for effectively applying this technique in different contexts.
Accuracy of the Method
This method can be quite accurate for identifying certain elements, particularly metals with well-established and distinctive flame colors. For example, metals like sodium and potassium have very characteristic emissions that are easy to recognize even with the naked eye. However, the accuracy of identification can be compromised if the sample contains multiple substances, as the colors may overlap or be less distinct. Additionally, environmental factors such as the presence of impurities in the sample can alter the flame color, further affecting the reliability of the results.
Limitations of the Method

Despite its advantages, there are several key limitations to the technique:
- Limited precision: The method is qualitative, not quantitative, which means it cannot provide detailed concentrations of elements in a sample.
- Interference from contaminants: The presence of other elements or compounds can cause overlapping colors or distortions, leading to inaccurate readings.
- Complex mixtures: When a sample contains a variety of elements, the resulting emissions may be too complex to easily interpret with this method.
- Color recognition: Identifying subtle color changes can be challenging, especially in poor lighting or with weak emissions from certain elements.
In conclusion, while this technique is useful for rapid, preliminary identification of elements, it is not always the most reliable for complex samples or precise measurements. It is best used in conjunction with other methods to confirm results and provide more comprehensive data.
Safety Protocols in Flame Test Laboratories
Ensuring safety in any experimental environment is crucial, especially when dealing with high temperatures, reactive substances, and open flames. In settings where substances are heated to identify their chemical composition, proper safety protocols are essential to prevent accidents and protect everyone involved. Adhering to established guidelines helps minimize risks and ensures the safe handling of materials and equipment.
When performing heating procedures, it is vital to follow strict safety measures. These protocols are designed to protect the individual conducting the experiment as well as anyone in the surrounding area. Below are key safety precautions that should be taken in any environment where heating methods are employed:
Essential Safety Measures
- Protective Gear: Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat to shield yourself from potential burns, splashes, or flying particles.
- Proper Ventilation: Ensure that the workspace is well-ventilated to prevent the accumulation of harmful fumes and gases, especially when working with volatile materials.
- Safe Handling of Chemicals: Be cautious when handling chemicals or substances that can react when exposed to heat. Always refer to the safety data sheets (SDS) for specific handling and disposal instructions.
- Flame Control: Maintain control over any flames or heat sources. Keep flammable materials at a safe distance, and never leave heat sources unattended.
- Fire Extinguishing Equipment: Ensure that a fire extinguisher is readily available and properly maintained in case of emergencies. Be familiar with how to use it in the event of a fire.
Work Area Maintenance
- Clean Workspace: Keep your workspace free from unnecessary clutter to avoid accidental spills or contact with flammable materials.
- Proper Waste Disposal: Dispose of waste materials, especially those that are hazardous or reactive, in designated containers according to safety regulations.
- Labeling and Storage: Store chemicals and reactive materials in clearly labeled containers. Ensure that they are kept in appropriate storage areas to avoid accidental reactions or exposure.
By following these safety protocols, individuals conducting experiments can significantly reduce the risk of accidents. It is always important to maintain awareness of potential hazards and to be prepared with the right tools and knowledge to manage any situation that might arise.
Flame Test Equipment and Setup
In any experimental procedure involving high-temperature reactions, having the right equipment and setting up the workspace properly are essential for successful results and safety. For this type of analysis, the setup involves specific tools and materials designed to facilitate controlled heating while ensuring the safety of everyone in the environment. The equipment required varies depending on the goals of the experiment, but there are a few common essentials for conducting safe and effective procedures.
Basic Equipment Needed
- Heat Source: A controlled heat source, such as a Bunsen burner or a propane torch, is essential for producing the necessary temperatures to cause the materials to react. The heat source must be adjustable to ensure precision in the experiment.
- Burner Stand: A stable stand is required to hold the heat source securely during experiments, minimizing the risk of accidents due to unstable equipment.
- Wire Loop: A metal wire loop, often made of platinum or nichrome, is used to hold the sample that will be heated. The wire must be resistant to high temperatures to avoid contamination or melting.
- Heat-resistant Surface: A heat-resistant surface or mat is necessary to place equipment and materials safely, preventing damage to the work area.
- Safety Equipment: Protective gear such as goggles, gloves, and lab coats is mandatory to prevent injuries from heat exposure, splashes, or accidental spills.
Setting Up the Experiment
- Prepare the Sample: Before starting, the sample should be properly prepared by placing it on the wire loop. It’s important to ensure that the sample is of the correct size and consistency for the experiment.
- Adjust the Heat Source: The heat source should be adjusted to the right intensity for the materials being tested. Ensure the flame is steady and not too large to prevent unnecessary risks.
- Position the Equipment: The wire loop with the sample should be positioned directly above the heat source, making sure it is within a safe distance to allow for controlled heating without causing splashing or unwanted reactions.
- Monitor the Reaction: During the experiment, continuously observe the material’s reaction to the heat. Keep a safe distance and be ready to extinguish the flame if needed.
Proper setup and equipment are crucial for the accuracy of results and the safety of those involved. A well-prepared environment allows for clear observation of reactions and ensures that any adjustments can be made quickly and efficiently, contributing to the overall success of the experiment.
Impact of Flame Test on Material Science
The study of materials through high-temperature reactions plays a pivotal role in the advancement of material science. By observing how certain substances react under specific conditions, researchers gain invaluable insights into the composition and properties of materials. This approach has led to significant developments in various fields, from metallurgy to environmental science, by providing a simple yet effective method to identify and classify materials based on their thermal responses. The ability to detect and analyze characteristic reactions helps experts understand the behavior of materials in different environments and applications.
Contribution to Material Identification
One of the most significant contributions of this technique to material science is its role in identifying chemical elements in unknown materials. By observing the specific color changes produced when a material is heated, researchers can quickly determine the presence of certain metal ions and compounds. This identification process aids in the analysis of minerals, alloys, and compounds, which is crucial in fields such as geology and metallurgy.
Applications in Research and Industry
In addition to its use in academic research, this method has found widespread applications in various industries. For example, in the production of metals and alloys, it helps ensure the correct composition of raw materials, which is essential for achieving the desired mechanical properties in finished products. Moreover, this technique is often used in quality control processes to verify material consistency and prevent defects in industrial applications.
Overall, the impact of this method on material science is profound, offering researchers a quick, cost-effective, and reliable tool for material characterization. It continues to serve as a foundation for both theoretical and applied research, contributing to innovations in manufacturing, environmental science, and engineering.
Flame Test Results for Educational Purposes
The study of materials through high-temperature reactions provides an excellent opportunity for hands-on learning in various educational settings. By observing how different substances behave when exposed to heat, students gain practical insights into chemical composition and molecular interactions. This simple yet effective demonstration enables learners to visually connect theoretical knowledge with real-world applications. Educational institutions often use this technique to foster curiosity and enhance understanding in subjects such as chemistry, materials science, and physics.
Enhancing Student Engagement
One of the key benefits of using this technique in the classroom is its ability to capture student interest. The vivid color changes produced by different materials stimulate curiosity and make the learning process more interactive and engaging. Students can easily grasp the concept of metal ion reactions and the principles of spectroscopy through direct observation. This hands-on experience helps to solidify abstract concepts and improve retention, as students are able to relate theoretical learning to tangible outcomes.
Applications in Educational Demonstrations
In educational settings, this method is frequently used for demonstrations that highlight the diversity of chemical reactions and the identification of materials based on their thermal responses. It allows students to explore the periodic table in a practical way, as they observe how different elements produce distinct colors when heated. These experiments are commonly conducted in chemistry labs to demonstrate the principles of atomic emission spectra and to reinforce the concept of electron excitation and de-excitation in atoms.
Furthermore, the technique is also valuable in understanding the safety and precautions involved in handling chemicals and conducting controlled experiments. Students learn the importance of safety protocols while simultaneously gaining an appreciation for the scientific method and critical thinking.
Recent Innovations in Flame Test Technology
Advancements in technology have significantly enhanced the accuracy, efficiency, and versatility of methods used to identify materials through high-temperature reactions. Recent innovations have introduced new tools, techniques, and improvements that make these procedures more accessible and effective, especially in both educational and industrial applications. These innovations not only streamline the testing process but also enable more precise results, ensuring a higher level of detail in material identification.
New Developments in Spectroscopy
One of the most notable breakthroughs in recent years is the improvement in spectroscopy methods that complement traditional high-temperature reactions. These innovations include:
- Portable Spectrometers: Handheld devices now allow for on-site analysis, eliminating the need for bulky equipment and enabling faster results in the field.
- High-Resolution Detectors: New detectors provide more detailed spectral data, allowing for more accurate identification of complex materials with minimal interference from background noise.
- Automated Data Processing: Sophisticated software has streamlined the process of analyzing and interpreting spectral data, reducing human error and providing more reliable conclusions.
Advances in Temperature Control
Recent improvements in temperature control systems have also played a crucial role in refining the precision of high-temperature reactions. With better regulation of heat sources, it’s now possible to more accurately replicate specific conditions that lead to distinctive material responses. Some of the key advancements include:
- Computer-Driven Heat Regulation: Advanced temperature controllers now allow for fine-tuned adjustments, maintaining a consistent and precise heat level throughout the reaction process.
- High-Temperature Resistant Materials: New materials used in the construction of heating elements allow for longer, more stable reactions without degradation, leading to consistent and repeatable results.
- Real-Time Monitoring: Continuous monitoring systems track temperature fluctuations in real time, ensuring that reactions occur under optimal conditions and reducing the risk of experimental error.
These developments make high-temperature material identification faster, more reliable, and applicable in a wider range of industries, including forensics, materials science, and environmental testing. As technology continues to advance, it is likely that even more precise and efficient methods will emerge, offering new possibilities for scientific discovery and material analysis.
Future of Flame Test Analysis in Industry
The evolving landscape of industrial applications for material identification techniques points to exciting new developments. As technology progresses, methods that utilize high-temperature reactions are expected to become more accurate, accessible, and efficient. This evolution is driven by advancements in sensor technology, automation, and data processing, which promise to transform how industries identify and categorize materials, ensuring quicker and more reliable results.
Integration with Automation and Robotics
The future of high-temperature reactions lies in their integration with automated systems. Industries are increasingly adopting robotic arms and automated systems to handle repetitive tasks, such as sample preparation and material handling. This will streamline the process, reduce human error, and increase throughput in industrial settings. Key innovations include:
- Robotic Handling: Robots equipped with advanced sensors can perform precise material placement and removal from high-temperature environments, enhancing both safety and efficiency.
- Automation of Temperature Control: Automated systems that adjust heat sources based on real-time data will enable even greater consistency and control over experimental conditions.
- Integration with Quality Control Systems: Automated systems will be able to feed real-time data directly into quality control processes, providing instant feedback and allowing for immediate corrective actions.
Advanced Data Processing and Machine Learning
Data analytics will play a central role in the future of material identification. With the help of machine learning algorithms and AI, it will become possible to analyze vast amounts of spectral and temperature data to identify patterns and correlations that were previously difficult to discern. Some developments include:
- AI-Powered Predictive Models: Machine learning models will analyze data to predict material behavior and identify unknown substances with greater accuracy, reducing the need for extensive trial-and-error testing.
- Cloud-Based Platforms: Data can be stored and accessed remotely through cloud platforms, allowing for easy sharing of results across teams and locations, making collaborative work more efficient.
- Real-Time Analysis: With the integration of AI, real-time analysis will provide immediate feedback during the material identification process, ensuring faster decision-making and reducing delays.
The future of material identification methods using high-temperature reactions will significantly enhance their application in industries such as manufacturing, environmental science, forensics, and quality control. These advancements will lead to faster, more reliable, and cost-effective processes, improving efficiency and safety in various sectors.