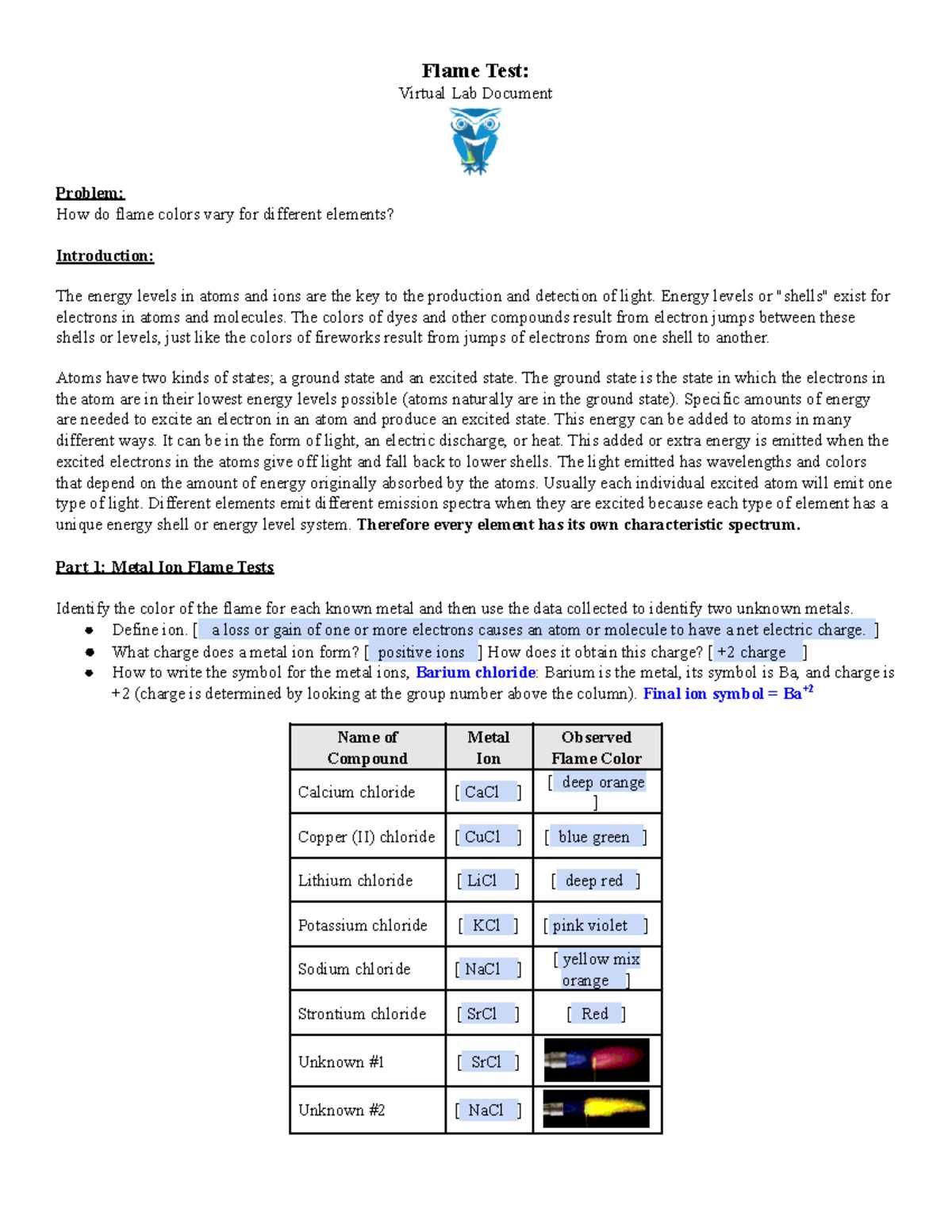
Flame Test Lab Answers and Key Results Explained

In the study of chemistry, observing the color shifts of substances when exposed to heat provides valuable insights into their composition. This process allows scientists to identify the presence of various elements based on the hues they emit under specific conditions. Such experiments play a key role in both educational and practical applications of chemistry, offering a simple yet powerful way to analyze materials.
Various elements produce distinctive shades when heated, each corresponding to specific wavelengths of light. These colors are linked to the transitions of electrons in atoms, a phenomenon that can be carefully studied to uncover the characteristics of unknown substances. By understanding these reactions, chemists can gather essential information about the materials they are working with, making this technique an invaluable tool in both research and industry.
Whether it’s determining the composition of a compound or testing for impurities, this method helps unravel the mysteries of matter at a molecular level. The next sections will explore how this process works, what results are expected, and the practical applications of these findings in various fields of science.
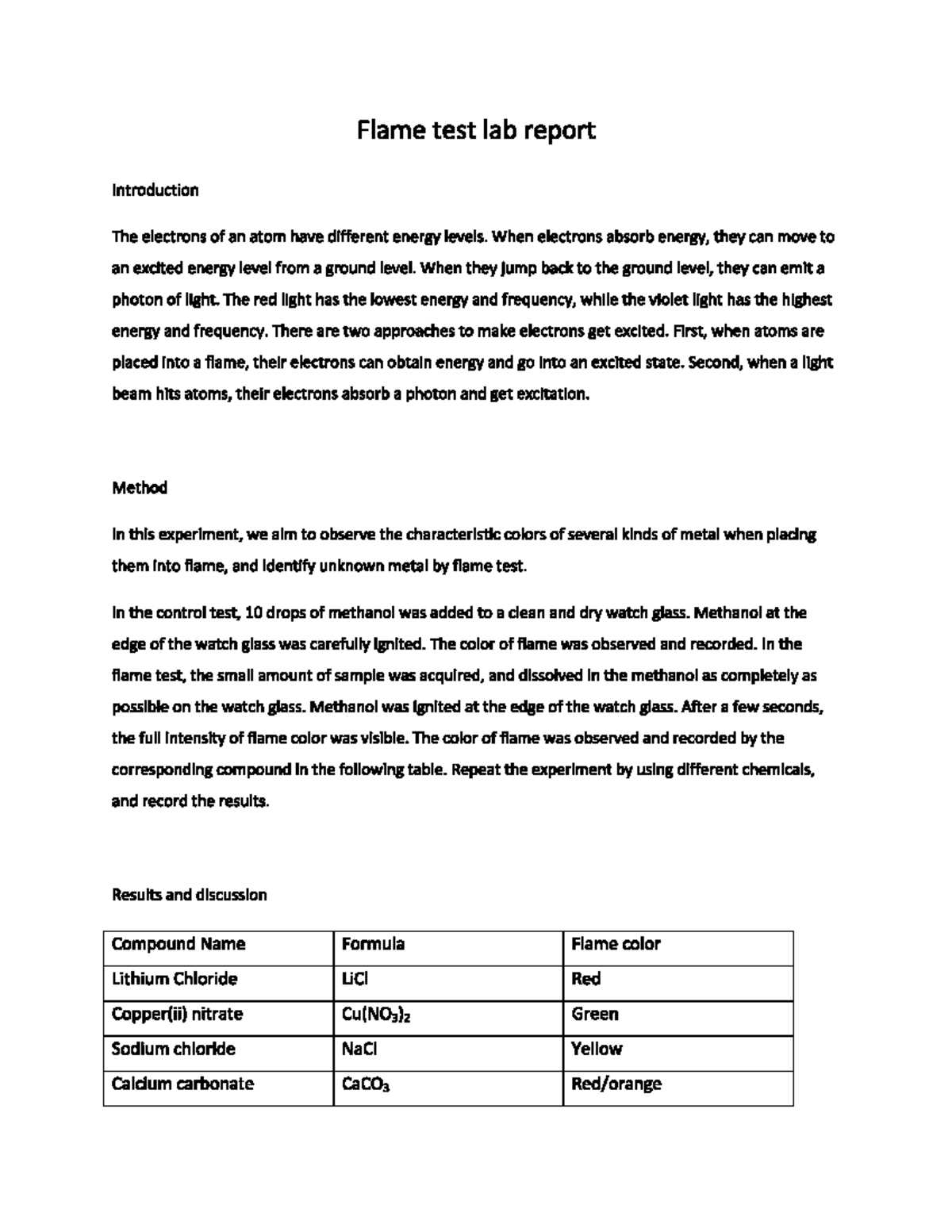
Flame Test Lab Answers
In chemical analysis, observing the behavior of materials when exposed to heat reveals important details about their atomic structure. This process is widely used to identify elements by the colors they emit, offering a straightforward method for distinguishing between substances. The reactions produced during heating provide key insights into the nature of the compounds under observation, allowing chemists to make informed conclusions about their composition.
Key Reactions and Results
The colors produced by different substances when heated correspond to the energy changes occurring within their atoms. Each element has a characteristic set of colors, making it possible to deduce the identity of the substance. For example, sodium produces a bright yellow hue, while copper emits a greenish-blue tone. These distinct color emissions are the result of electrons jumping between energy levels and releasing photons of specific wavelengths.
Practical Applications in Chemistry
This method of material analysis is not only useful in educational settings but also plays a crucial role in various industrial and research applications. From quality control in manufacturing to the identification of compounds in geological samples, observing color changes under heat is an essential tool in many scientific fields. Understanding the principles behind these reactions enhances our ability to analyze and manipulate chemical substances in both controlled and real-world environments.
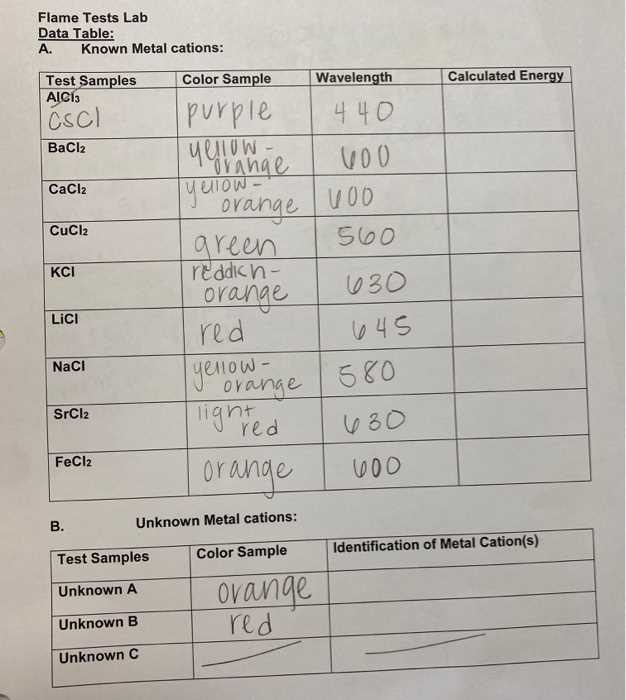
Understanding the Flame Test Procedure
In chemical analysis, observing how substances behave when exposed to heat provides valuable insights into their composition. This process involves heating a sample and observing the color emitted, which is directly related to the atomic structure of the material. The procedure is simple yet effective in identifying various elements based on the distinctive colors they produce under heat.
Steps Involved in the Process
The procedure typically begins with a clean metal wire, often made of platinum or nichrome, which is used to hold the sample. The wire is first dipped into a solution containing the substance to be tested and then placed into a heated flame. As the sample heats up, electrons in the atoms of the material gain energy and move to higher energy levels. When they return to their original positions, they release energy in the form of light, producing a specific color.
Key Considerations for Accurate Results

To obtain accurate results, it is essential to ensure the wire is free of contaminants that could affect the color emitted by the sample. In addition, the intensity and purity of the flame should be consistent to avoid misleading results. A strong and steady flame helps in observing clear and distinct color emissions, which are crucial for identifying the elements present in the sample.
Common Elements Tested in Flame Tests
Various elements are frequently analyzed through heating, as each produces a unique color when subjected to high temperatures. These distinct color emissions are a direct result of the behavior of electrons within the atoms of the element. By observing these colors, chemists can identify the substances present in a sample without needing complex equipment.
Sodium and its Characteristics
Sodium is one of the most commonly tested elements due to the bright yellow flame it produces. This characteristic color is easily recognizable and is often used as a reference in identifying other elements. Sodium’s emission spectrum is particularly useful in both educational and professional settings for quick and effective identification.
Potassium and its Violet Emission
Potassium, when heated, produces a light violet or lilac flame. This distinctive color is another useful tool in the identification of substances. The emission spectra of potassium can be less intense than sodium’s, but its unique shade makes it easy to distinguish in a sample.
What Is a Flame Test Reaction?
A reaction involving the heating of substances to observe the color emitted is a key method for identifying chemical elements. When a material is exposed to high temperatures, the atoms or ions within it absorb energy. This energy causes electrons to move to higher energy levels. As the electrons return to their original positions, they release the absorbed energy in the form of light, producing a distinct color that corresponds to the element’s unique energy transitions.
The Role of Electrons in Color Emission
The color seen during such reactions depends on the specific energy gaps between electron orbitals in different elements. These transitions determine the wavelength of light emitted. The process can be summarized as follows:
- The substance is heated in a high-temperature environment.
- Electrons in the atoms absorb energy and jump to higher energy levels.
- When electrons return to their lower energy states, they release energy in the form of light.
- The wavelength of the emitted light corresponds to a specific color visible to the naked eye.
Why It Matters for Chemical Identification

This reaction is important because different elements emit different colors due to their unique electron configurations. The precise color observed provides a fingerprint for the element in question, allowing scientists to identify substances based on their spectral emission. This simple yet powerful reaction is widely used in laboratories for educational purposes and practical applications like material testing and analysis.
Significance of Flame Colors in Chemistry
The color produced when a substance is heated is not just a visual effect, but a powerful indicator of its atomic composition. Each element emits a specific color when subjected to high temperatures, due to the unique way its electrons absorb and release energy. These colors are crucial for identifying and differentiating between elements in various chemical and industrial processes.
Understanding the Emission Spectra
The emission spectra of elements are essentially the light patterns produced when electrons return to lower energy states after being excited by heat. Each element has its own characteristic spectrum, which can be used to identify its presence in a sample. The color emitted depends on the size of the energy gaps between the electron shells of the element. The following table summarizes the flame colors produced by common elements:
| Element | Flame Color |
|---|---|
| Sodium | Bright Yellow |
| Potassium | Light Violet |
| Calcium | Orange-Red |
| Copper | Green-Blue |
| Strontium | Crimson Red |
Applications in Chemical Analysis
By observing these characteristic flame colors, scientists can quickly identify the elements present in a sample without the need for complex equipment. This simple but effective method is commonly used in both educational settings and professional laboratories for qualitative analysis. The ability to recognize these colors enables chemists to identify unknown substances, check for impurities, and perform various other tests in fields ranging from materials science to environmental monitoring.
How to Conduct a Flame Test
Performing a heating procedure to observe the colors emitted by a sample is a simple yet effective way to identify chemical elements. The process involves heating a small amount of a substance and observing the characteristic color it produces. This method relies on the unique emission spectra of different elements, making it a useful tool for chemical analysis.
Required Materials
Before starting the procedure, ensure that you have the following materials on hand:
- Metal wire (platinum or nichrome is commonly used)
- Heat source (Bunsen burner or alcohol lamp)
- Substance to be tested (usually in solution form)
- Clean beaker or container (for holding the substance)
- Safety equipment (goggles, gloves, and lab coat)
Steps for Conducting the Procedure
Follow these steps to properly conduct the procedure:
- Clean the metal wire thoroughly to remove any contaminants that could interfere with the results.
- Dip the clean wire into the solution of the substance you wish to test.
- Place the wire with the sample into the heat source and observe the color emitted by the substance.
- Record the color and compare it to known reference colors for element identification.
- For accuracy, repeat the procedure with a fresh sample if needed.
Ensure safety protocols are followed throughout the procedure. Use the appropriate protective equipment, and work in a well-ventilated area to minimize exposure to any harmful fumes. This method provides valuable insights into the elemental composition of substances and is widely used in various scientific applications.
Factors Affecting Flame Test Results

The accuracy of identifying elements through heating can be influenced by several variables. While this method is generally reliable, certain factors can alter the color emitted, making interpretation more challenging. Understanding these factors is crucial for ensuring precise results and avoiding misidentification of substances.
One key factor is the purity of the sample being tested. Contaminants, even in small amounts, can affect the color emitted, leading to misleading results. Additionally, the intensity and consistency of the heat source play a significant role. A fluctuating or insufficiently heated flame may not excite the electrons in the sample properly, resulting in weak or incorrect colors.
Another important factor is the concentration of the substance being tested. A higher concentration typically produces a more vivid color, while a very dilute sample may lead to faint emissions that are harder to distinguish. The presence of moisture or other volatile substances in the sample can also interfere with the reaction, as water vapor can absorb energy and reduce the intensity of the color observed.
Environmental conditions such as room temperature, humidity, and even the type of container used can also impact the results. To achieve the best possible outcome, it is important to control these variables as much as possible and ensure that the procedure is performed under consistent and optimal conditions.
Why Do Different Elements Have Unique Colors?
The distinctive colors emitted by elements when heated are a direct result of their atomic structure. Each element has a unique arrangement of electrons orbiting the nucleus, and when these electrons are excited by heat, they absorb energy and jump to higher energy levels. As they return to their original positions, they release energy in the form of light. The wavelength of this emitted light corresponds to a specific color, and the color produced depends on the size of the energy gaps between the electron shells of the element.
The Role of Electron Transitions
When atoms are heated, their electrons gain enough energy to jump to higher orbitals. The energy difference between these orbitals determines the amount of energy released as the electrons fall back to their original positions. This energy is emitted as light, and the wavelength of that light defines the color we observe. Since different elements have different electron configurations, the energy levels and gaps between orbitals vary, resulting in unique colors for each element.
Why Some Elements Have Similar Colors
While each element has a characteristic color, elements within the same group on the periodic table often produce similar colors. This is due to their similar electron configurations, which result in similar energy transitions. For example, alkali metals like sodium and lithium emit bright colors, but their exact shades differ because of subtle differences in their atomic structures.
Role of Electrons in Flame Tests
The behavior of electrons plays a fundamental role in the process that allows for the identification of elements through color emissions. When a substance is heated, the energy provided causes its atoms or ions to undergo electronic transitions. These transitions are key to understanding how different elements emit distinct colors when subjected to high temperatures.
When energy is supplied to an atom, the electrons within the atom absorb that energy and move from a lower energy state to a higher one. This state is often unstable, and the electron eventually returns to its original, lower energy level. As the electron drops back to its previous state, it releases the excess energy in the form of light. The specific wavelength, or color, of this light depends on the difference in energy levels between the excited and ground states of the electron.
Each element has a unique set of energy levels, determined by its atomic structure. Therefore, the light emitted when electrons transition between these levels has a specific color associated with it. This is why each element produces a characteristic color when heated. The role of electrons in these transitions is essential, as their movement between energy states is what ultimately produces the visible light that can be observed and used for identification purposes.
Flame Test and Chemical Identification
One of the most useful methods for identifying elements in a substance is through the observation of the colors produced when the material is heated. This process relies on the fact that each element emits a characteristic color when its atoms are excited by heat. These colors can then be compared to known standards to determine the identity of the chemical present.
Identification Based on Color Emission
Each element has a unique atomic structure, and the electrons in the atoms of different elements occupy different energy levels. When the material is heated, electrons in the atoms absorb energy and jump to higher energy levels. As they return to their ground state, they release energy in the form of light, with the wavelength (and thus the color) of this light being specific to the element. By observing the emitted color, scientists can identify the element present in the sample.
Applications in Chemical Analysis
This method is widely used in various scientific fields for rapid and preliminary identification of substances. It is especially useful in chemistry for detecting specific metals in samples. In laboratories, this technique provides a quick and non-destructive way to identify chemical components, aiding in everything from environmental testing to forensic investigations.
Flame Test Results for Sodium

Sodium is a well-known element that produces a distinctive color when exposed to high temperatures. When heated, sodium atoms undergo electronic transitions that result in the emission of light. This light is characteristic of sodium and is often used as a key identifier in chemical analysis.
The color emitted by sodium during heating is typically a bright yellow. This yellow color is the result of a specific electron transition within the sodium atoms. When sodium is heated, its electrons move to higher energy levels, and as they return to their original positions, they release energy in the form of light. The wavelength of this light corresponds to the yellow color observed, making it easily recognizable in laboratory experiments.
This unique yellow emission is often used as a diagnostic tool for identifying sodium in various chemical samples. It is one of the most consistent and well-documented results, making it an essential part of chemical identification procedures in educational and research settings.
Flame Test Results for Potassium
Potassium, like many other elements, exhibits a unique color when subjected to heat. The color produced by potassium is a result of the excitation of its electrons, which absorb energy and then release it as light when they return to their original energy states. This light is specific to potassium, making it easily identifiable in various chemical analyses.
Characteristic Violet Color
When heated, potassium emits a pale violet or light purple color. This emission is the result of electron transitions within the potassium atom. As the atom’s electrons are excited by the heat, they jump to higher energy levels, and as they fall back to their ground state, they release energy in the form of visible light. The wavelength of this light corresponds to the violet hue, which is a distinctive feature for identifying potassium in samples.
Importance in Chemical Identification
The violet color emitted by potassium is an important tool in the identification of this element. It is frequently used in laboratories to distinguish potassium from other elements that produce different colors when heated. This method provides a quick, reliable, and non-destructive means of analysis, making it valuable for chemical tests in both educational and professional settings.
Flame Test Results for Calcium
Calcium is another element that displays a characteristic color when subjected to high temperatures. This color is produced due to the electronic transitions of calcium atoms, where the energy absorbed during heating is released in the form of light. The specific color emitted is unique to calcium, allowing it to be easily identified in a variety of chemical applications.
Characteristic Orange-Red Emission

When calcium is heated, it produces a brick-red or orange-red glow. This is a result of the specific wavelengths of light emitted as the electrons in calcium atoms return to their ground state after being excited by heat. The orange-red hue is consistent and distinct, making it a reliable feature for identifying calcium in samples.
Significance in Elemental Identification

The orange-red emission from calcium is often used in analytical techniques to quickly identify the presence of calcium ions. Some of the key applications include:
- Analyzing mineral compositions
- Identifying calcium in biological samples
- Verifying the presence of calcium in industrial materials
This color emission method is invaluable for both qualitative and quantitative analysis, allowing for accurate identification in various scientific and educational settings.
Safety Guidelines for Flame Testing

When conducting experiments involving high heat or open flames, it is crucial to follow safety procedures to prevent accidents and ensure the well-being of everyone in the environment. Proper precautions can minimize the risk of injury or damage during chemical procedures that involve intense heat or combustion. By adhering to established safety protocols, you can create a secure setting for conducting experiments and handling materials safely.
General Safety Measures

Before starting any experiment involving high temperatures, make sure to:
- Wear protective gear such as heat-resistant gloves, goggles, and a lab coat to shield yourself from potential hazards.
- Ensure proper ventilation in the workspace to prevent the buildup of harmful gases or fumes that may be emitted during heating processes.
- Keep flammable materials away from the heat source and ensure that no combustible substances are nearby.
- Work in a controlled environment such as a fume hood or a well-ventilated area to reduce exposure to any potentially harmful substances.
Handling Chemicals and Heat Sources
When working with heat sources, it is essential to:
- Use proper equipment like a Bunsen burner or a similar controlled flame source, ensuring that all connections are secure and functioning properly.
- Monitor the experiment closely and avoid leaving the heat source unattended to prevent accidents.
- Dispose of materials safely after the experiment, ensuring that no leftover chemicals are exposed to heat or improperly discarded.
By adhering to these guidelines and staying vigilant throughout the process, you can ensure a safe and productive environment while conducting experiments that involve heating substances for analysis or identification.
How Flame Tests Aid in Analytical Chemistry
In analytical chemistry, identifying the chemical composition of substances is a critical step for understanding their properties and behavior. One method widely used to reveal the elemental makeup of compounds involves exposing them to high temperatures. The distinct colors emitted during this process can provide valuable insights into the types of elements present. This approach is particularly useful for quick, qualitative analysis in both laboratory and field settings.
Applications of This Method
This technique offers numerous advantages in various fields of chemistry:
- Identification of metal ions: Different metals produce specific colors when heated, allowing chemists to identify them based on their emission spectra.
- Fast and cost-effective: The process is relatively inexpensive and does not require complex instrumentation, making it accessible for routine analyses.
- Educational tool: This method is often used in classrooms to demonstrate fundamental concepts of atomic structure and electron behavior.
How Results Assist Chemists

By carefully observing the color changes, chemists can draw conclusions about the chemical composition of a sample. These results play a crucial role in:
- Qualitative analysis: Determining the presence of certain elements in unknown samples or mixtures.
- Forensic investigations: Identifying trace elements in forensic samples, helping to solve criminal cases or environmental studies.
- Environmental testing: Analyzing soil, water, or air samples for contamination by heavy metals or other pollutants.
In summary, this technique is an essential tool in analytical chemistry, offering a simple yet effective way to analyze and identify substances based on their elemental composition.
Limitations of Flame Test Analysis
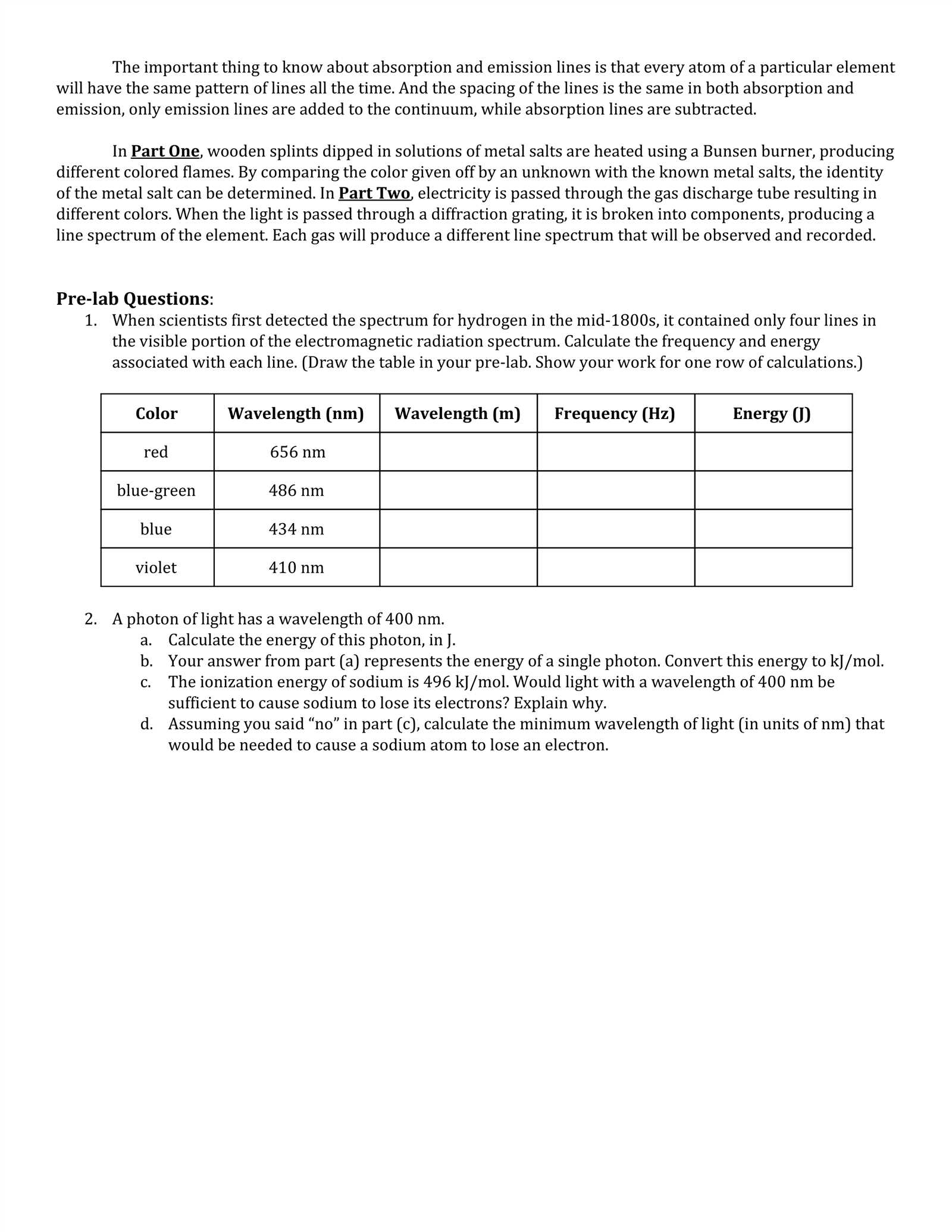
While this analytical technique is widely used for identifying certain elements based on the colors they emit when heated, it has several limitations that can affect its accuracy and reliability. These drawbacks can limit its application in more complex analyses or situations that require precise results. Understanding these constraints is essential for choosing the right method for elemental analysis in various scientific fields.
Some of the key limitations of this approach include:
| Limitation | Impact |
|---|---|
| Limited to metal ions: | This technique is primarily effective for identifying metal ions, making it unsuitable for detecting non-metallic elements or compounds. |
| Interference from other substances: | When multiple elements are present, the colors emitted may overlap, making it difficult to distinguish between them accurately. |
| Qualitative rather than quantitative: | The analysis is generally qualitative, offering only the presence or absence of certain elements, but not their exact concentrations. |
| Background contamination: | Contaminants in the sample or surrounding environment can skew results, leading to false positives or inaccurate readings. |
| Requires a clean, controlled environment: | Results can be compromised by external factors such as the type of flame or impurities in the heating source. |
Despite these limitations, the method remains useful in various applications where quick, general identification is needed. However, for more precise and comprehensive analysis, additional techniques may be required to confirm the findings and provide detailed results.
Advanced Techniques Beyond Flame Testing
While traditional methods of elemental identification based on the emission of colored light are widely used, they are often limited in their accuracy, sensitivity, and applicability. In more complex analyses, scientists turn to advanced techniques that provide more precise results and can be applied to a wider range of materials. These methods go beyond basic color emission and can offer quantitative data, multi-element analysis, and enhanced reliability in various scientific and industrial applications.
Some advanced techniques that are commonly used include:
- Atomic Absorption Spectroscopy (AAS): AAS is a powerful method for detecting the concentration of elements in a sample. Unlike emission-based techniques, AAS measures the absorption of light by atoms in a sample, offering greater sensitivity and accuracy.
- Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES): ICP-OES allows for the simultaneous detection of multiple elements in a sample by measuring the light emitted from excited atoms. It is particularly useful for trace element analysis in complex mixtures.
- Mass Spectrometry (MS): This technique provides high precision and sensitivity for elemental and molecular analysis. It involves ionizing a sample and measuring the mass-to-charge ratio of the resulting ions, allowing for detailed elemental and isotopic analysis.
- X-ray Fluorescence (XRF): XRF is a non-destructive technique that allows for rapid elemental analysis. It works by irradiating a sample with high-energy X-rays and detecting the fluorescent X-rays emitted from the elements within the sample.
- Raman Spectroscopy: Raman spectroscopy provides information about molecular vibrations and can be used to identify elements and compounds based on their unique vibrational modes. It is highly effective for chemical analysis of solid, liquid, and gas samples.
These techniques offer a significant advantage over traditional methods by providing more detailed, accurate, and multi-faceted data. They are particularly useful in fields such as environmental monitoring, forensic analysis, pharmaceutical research, and materials science, where precision and comprehensive analysis are essential.